Suzuki
Examples:
Example 1

The Iodo compound (308 mg, 0.513 mmol), the boronic ester (194 mg, 0.641 mmol), K3PO4 (327 mg, 1.539 mmol), XPhos (14.67 mg, 0.031 mmol), and Pd2(dba)3 (14.09 mg, 0.015 mmol) were combined in a microwave vial. The vial was purged with argon, after which time was added dioxane (3 mL) and H2O (0.5 mL) The reaction was irradiated in a microwave reactor at 120 C for 10 min. Additional boronic ester (50 mg) was added and the mixture was irradiated at 120 C for another 10 min. The mixture was then treated with aq 1N NaOH and extracted with EtOAc (50 mL). The org layer was dried (MgSO4), concentrated, and purified by flash chromatography (20% MeOH/DCM) to provide the product as a dark yellow solid. [177 mg, 53%]
[Patent Reference: WO2012129338, page 69, ![]() (12.0 MB)]
(12.0 MB)]
Example 2
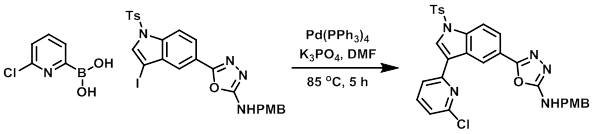
To a solution of the Iodo compound (1.0 g, 1.66 mmol) and the boronic acid (392 mg, 2.49 mmol) in DMF (10 mL) was added K3PO4 (704 mg, 3.32 mmol). The mixture was purged with N2 for 15 min, after which time Pd(PPh3)4 (176 mg, 0.152 mmol) was added and the mixture was stirred at 85 C for 5 h. The mixture was then poured in ice cold H2O (50 mL) and extracted with EtOAc. The combined organics were washed with H2O, dried, concentrated, and purified by silica gel chromatography (eluting with 15% EtOAc/hexane) to provide the product as a brown solid. [0.52 g, 53%]
[Patent Reference: WO2012129338, page 79, ![]() (12.0 MB)]
(12.0 MB)]
Example 3
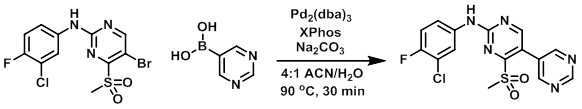
A suspension of the bromide (400 mg, 1.05 mmol), the boronic acid (208 mg, 1.68 mmol), Pd2(dba)3 (96 mg, 0.1 mmol), XPhos (148 mg, 0.3 mmol), and Na2CO3 (112 mg, 1.05 mmol) in ACN/H2O (4:1) was heated at 90 C for 30 min. The reaction mixture was diluted with EtOAc (10 mL), the org layer was separated, dried (Na2SO4), and concentrated in vacuo. The crude was purified by column chromatography (1:9 MeOH/CHCl3) to provide the product. [20%]
[Patent Reference: WO2010038081, page 109, ![]() (33.8 MB)]
(33.8 MB)]
Example 4
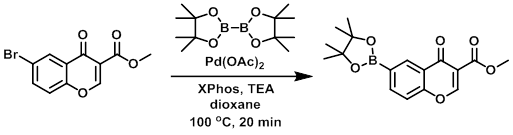
A mixture of the SM (400 mg, 1.41 mmol), bis(pinacolato)diboron (1 g, 3.94 mmol), XPhos (135 mg, 0.28 mmol), Pd(OAc)2 (16 mg, 0.06 mmol), and TEA (570 mg, 5.6 mmol) in dioxane (20 mL) was degassed and refluxed at 100 C for 20 min under N2. The solvent was removed in vacuo, the residue diluted with DCM (20 mL), washed with H2O (2 x 20 mL), then brine. The org layer was dried (Na2SO4), concentrated, and recrystallized with DCM/hexane to provide the product. [300 mg]
[Patent Reference: WO2010038081, page 129, ![]() (33.8 MB)]
(33.8 MB)]
Example 5
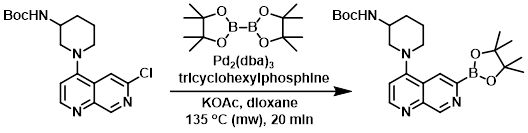
A solution of the SM (1.0 equiv), KOAc (3.0 equiv), bis(pinacolato)diboron (2.0 equiv), tricyclohexylphosphine (0.8 equiv), and Pd2(dba)3 (0.2 equiv) in dioxane (0.06 M) was heated at 135 C in a microwave reactor for 20 min. The reaction mixture was filtered through a 1 uL HPLC filter, concentrated, and dried to provide the product which was used without further purification.
[Patent Reference: WO2010026121, page 36, ![]() (3.6 MB)]
(3.6 MB)]
Example 6

The boronic ester (0.043 g, 0.16 mmol), the bromide (0.10 g, 0.16 mmol), and dioxane (1.6 mL) were combined to give a yellow suspension. A solution of Cs2CO3 (0.134 g, 0.411 mmol) in H2O (0.4 mL) was added, then N2 was bubbled through the mixture. Pd(Ph3)2Cl2 (0.008 g, 0.012 mmol) was added and the mixture was flushed again with N2. The reaction mixture was stirred at 75 C for 18 h. The mixture was concentrated and the resulting residue was purified by silica gel chromatography (20-70% EtOAc/DCM) to provide the product. [0.072 g, 66%]
[Patent Reference: WO2012149280, page 80, ![]() (4.1 MB)]
(4.1 MB)]
Example 7
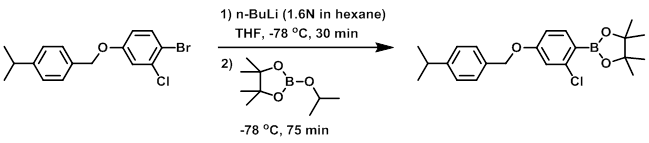
To a mixture of the SM (2.34 g, 6.48 mmol) in anhydrous THF (30 mL) at -78 C under N2 was added n-BuLi (1.6N in hexane, 4.25 mL, 6.8 mmol). The mixture was stirred at -78 C for 30 min, after which time was added isopropoxyboronic acid pinacol ester (1.36 mL, 6.67 mmol). The reaction mixture was stirred at -78 C for 75 min. The mixture was quenched with H2O then extracted with EtOAc. The org layer was washed with brine, dried (MgSO4), and concentrated to give 2.37 g of crude material. The material was purified by Prep LC (120 g silica, 0-20% EtOAc/heptane) to give 1.91 g of material. The material was repurified by Prep LC (50 g silica, 0-20% EtOAc/heptane) to provide the product. [1.12 g, 45%]
[Patent Reference: WO2015144799, page 123, ![]() (18.8 MB)]
(18.8 MB)]
Example 8
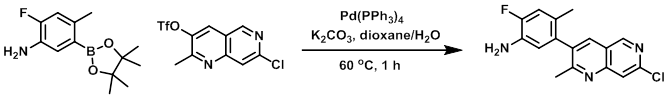
A mixture of the triflate (2.25 g, 6.89 mmol) and the boronic ester (1.73 g, 6.89 mmol) in dioxane (32 mL) was sparged with argon. To the mixture was then added K2CO3 (2.86 g, 20.7 mmol) in H2O (16 mL), followed by Pd(PPh3)4 (252 mg, 0.689 mmol). The reaction mixture was stirred at 60 C for 1 h. The mixture was cooled to RT and diluted with EtOAc. The mixture was successively washed with H2O, sat aq NaHCO3, and brine, dried (Na2SO4), and concentrated. The resulting material was purified by silica gel chromatography (EtOAc/hexane) to provide the product as a solid. [1.277 g, 61%]
[Patent Reference: WO2013134298, page 46, ![]() (4.1 MB)]
(4.1 MB)]
Example 9
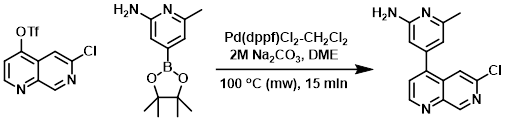
A solution of the triflate (1.0 equiv), the boronic ester (1.0 equiv), and Pd(dppf)Cl2-CH2Cl2 (0.1 equiv) in 3:1 DME/2M Na2CO3 was heated in a microwave reactor at 100 C for 15 min. Upon cooling, the solution was partitioned between EtOAc and sat aq Na2CO3. The org layer was separated and washed with sat aq NaCl, dried (MgSO4), concentrated, and purified by flash chromatography [2-4% MeOH/CH2Cl2 (w/ 0.1% DIEA)] to provide to product. [17%]
[Patent Reference: WO2010026121, page 38, ![]() (3.6 MB)]
(3.6 MB)]
