Magnesium Sulfate
General Information:
Structure:
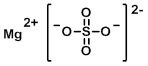
CAS Number: 7487-88-9 (anhydrous)
Molecular Weight: 120.37 g/mol (anhydrous)
Appearance: White solid
Chemical Formula: MgSO4
Anhydrous magnesium sulfate (MgSO4) is commonly used as a drying agent in organic chemistry. It is typically used to dry the organic layer after an aqueous work-up. Anhydrous magnesium sulfate is added to the wet organic layer and the resulting solid magnesium sulfate hydrate is simply removed by filtration, providing a dry (anhydrous) organic filtrate. Anhydrous sodium sulfate (Na2SO4) is the other drying agent that is commonly used for similar purposes.
Common Uses:
Drying agent for organic solvents (removes H2O)
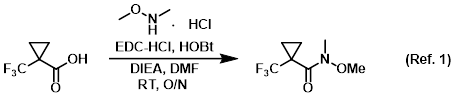
Procedure excerpt:
. . . The org layer was washed with H2O, brine, dried (MgSO4), and concentrated in vacuo to provide the product as a pale yellow oil. . . .
References:
1) Patent Reference: WO2015129926, page 93, ![]() (21.5 MB)
(21.5 MB)
2) Wikipedia: Magnesium sulfate (link)
3) www.sigmaaldrich.com: Magnesium sulfate (link)
