DMF
(Dimethylformamide)
Other Names:
N,N-Dimethylformamide
General Information:
Structure:
![]()
CAS Number: 68-12-2
Molecular Weight: 73.09 g/mol
Appearance: Colorless liquid
Melting Point: -61 C
Boiling Point: 153 C
Density: 0.944 g/mL
Dimethylformamide (DMF) is a polar aprotic solvent with a high boiling point (153 C). DMF is also used as a reagent in some reactions. A closely related solvent is dimethylacetamide (DMA).
Common Uses:
Catalytic reagent used with oxalyl chloride to convert carboxylic acids to acid chlorides
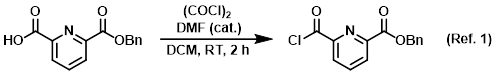
Reagent used for formylation reactions

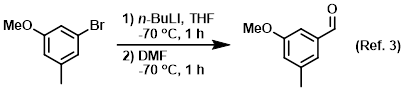
Solvent for reactions
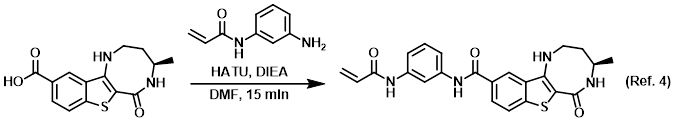
Safety:
Reactions using NaH in DMF can have sudden exotherms. This can be dangerous, especially on large scale. Runaway reactions have taken place between NaH and DMF. Under acidic or basic conditions, DMF is known to disproportionate to carbon monoxide and dimethylamine.
References:
1) Patent Reference: WO2016011390, page 126, ![]() (20.2 MB)
(20.2 MB)
2) Patent Reference: WO2015088045, page 146, ![]() (10.3 MB)
(10.3 MB)
3) Patent Reference: WO2016014463, page 93, ![]() (6.7 MB)
(6.7 MB)
4) Patent Reference: WO2014149164, page 261, ![]() (23.7 MB)
(23.7 MB)
5) Wikipedia: Dimethylformamide (link)
6) www.sigmaaldrich.com: N,N-Dimethylformamide (link)
7) Anderson, N. G.; Practical Process Research and Development, a Guide for Organic Chemists, 2nd Edition
