Chloroform
Other Names:
Trichloromethane
Methylidyne trichloride
General Information:
Structure:
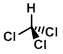
CAS Number: 67-66-3
Molecular Weight: 119.38 g/mol
Appearance: Colorless liquid
Chemical Formula: CHCl3
Melting Point: -63 C
Boiling Point: 61 C
Density: 1.489 g/mL
Chloroform (CHCl3) is a relatively common organic solvent. In most organic chemistry labs, however, methylene chloride is used as a substitute. Deuterated chloroform (CDCl3) is one of the most common NMR solvents.
Common Uses:
Solvent in bromination reactions
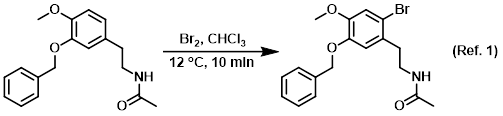
Procedure excerpt:
To a solution of the SM (80 g, 0.27 mol) in CHCl3 (1 L) at 10-15 C was added dropwise a solution of Br2 (16 mL, 0.31 mol) in CHCl3 (100 mL). The reaction was stirred . . .
Solvent in manganese dioxide (MnO2) oxidations (ex. alcohols to ketones)
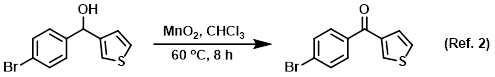
Procedure excerpt:
To a solution of the SM (100 g, 371.5 mmol) in CHCl3 (200 mL) was added MnO2 (322.9 g, 3.715 mol). The reaction mixture was stirred at 60 C for 12 h. After cooling to RT . . .
Solvent for silica gel chromatography
Procedure excerpt:
…The crude residue was purified by silica gel column chromatography (0-1% MeOH/CHCl3) to provide the product as a light yellow solid…
Solvent for extractions
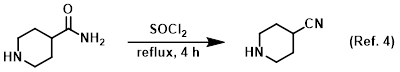
Procedure excerpt:
…was concentrated to a residue and partitioned between H2O and CHCl3. The layers were separated and the aq layer was further extracted with CHCl3 (4x)…
Safety:
Chloroform (CHCl3) is dangerous due to its ability to depress the central nervous system. In fact, chloroform was once commonly used as an anesthetic for just these CNS depressing effects. A fatal oral dose can be a small as 10 mL. Care should be taken when working with large amounts of chloroform.
References:
1) Patent Reference: WO2014177977, page 64, ![]() (6.0 MB)
(6.0 MB)
2) Patent Reference: WO2015140133, page 90, ![]() (11.7 MB)
(11.7 MB)
3) Patent Reference: WO2011014535, page 46, ![]() (17.4 MB)
(17.4 MB)
4) Patent Reference: WO2010104899, page 155, ![]() (6.7 MB)
(6.7 MB)
5) Wikipedia: Chloroform (link)
6) www.sigmaaldrich.com: Chloroform (link)
