Bromination
(SEAr)
Examples:
Example 1
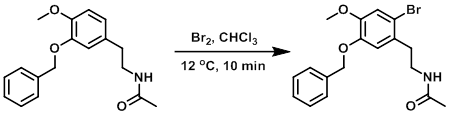
To a solution of the SM (80 g, 0.27 mol) in CHCl3 (1 L) at 10-15 C was added dropwise a solution of Br2 (16 mL, 0.31 mol) in CHCl3 (100 mL). The reaction was stirred at 12 C for 10 min, then washed sequentially with cooled sat aq NaHCO3 (2 x 500 mL) and sat aq brine (400 mL), dried, and concentrated in vacuo. Crystallization of the residue from EtOAc provided the product as a yellow solid. [90 g, 88%]
[Patent Reference: WO2014177977, page 64, ![]() (6.0 MB)]
(6.0 MB)]
Example 2

To a solution of the SM (603 mg, 2.75 mmol) in AcOH (27 mL) was added dropwise Br2 (0.15 mL, 2.92 mmol). The resulting mixture was stirred at RT for 2 h. The resulting solids were filtered and washed with AcOH (20 mL), EtOAc (30 mL), ether (4 x 7 mL), DCM (55 mL), saturated aq NaHCO3 (15 mL), and finally H2O (10 mL) to provide the product as a yellow solid. [718 mg]
[Patent Reference: WO2012112946, page 216, ![]() (11.2 MB)]
(11.2 MB)]
Example 3
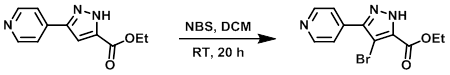
To a solution of the SM (34.7 g, 160 mmol) in DCM (464 mL) was added NBS (31.3 g, 176 mmol). The reaction mixture was stirred at RT for 20 h. The mixture was concentrated in vacuo, diluted with ether (200 mL), and filtered through a glass frit. The resulting solids were washed with ether (100 mL) and twice with MeOH/ether (10 mL/40 mL). The solids were dried in vacuo to provide the product. [43.57 g, 92%]
[Patent Reference: WO2015144799, page 84, ![]() (18.8 MB)]
(18.8 MB)]
Example 4

To a solution of the SM (4.7 g, 17 mmol) in ACN (60 mL) was slowly added NBS (3.0 g, 17 mmol) in ACN (60 mL) at RT. The reaction mixture was stirred at 80 C overnight. The mixture was concentrated in vacuo and the resulting material was taken up in EtOAc, washed with sat NaCl, NaHCO3, dried, concentrated, and purified by Prep LC (120 g, 2% EtOAc/heptane) to give 3.0 g of material. The material was repurified by Prep LC (40 g silica, 2% EtOAc/heptane) to provide the product (80% purity) as a colorless oil. [2.6 g, 47%]
[Patent Reference: WO2015144799, page 163, ![]() (18.8 MB)]
(18.8 MB)]
Example 5
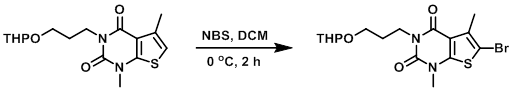
To a solution of the SM (3.0 g, 8.14 mmol) in DCM (30 mL) at 0 C was added NBS (1.6 g, 8.95 mmol). The reaction was stirred at 0 C for 2 h. The mixture was diluted with DCM (100 mL) and washed with H2O (2 x 50 mL). The org layer was dried (Na2SO4) and concentrated. The resulting residue was purified by chromatography (1:1 PE/EtOAc) to provide the product as a white solid. [3.4 g, 93%]
[Patent Reference: WO2016023832, page 50, ![]() (3.2 MB)]
(3.2 MB)]
Example 6
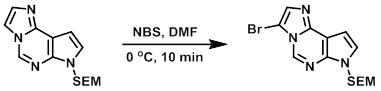
A mixture of the SM (1.02 g, 3.54 mmol) and DMF (22 mL) was cooled to 0 C. NBS (0.566 g, 3.18 mmol) was added and the reaction was stirred for about 10 min. The resulting precipitate was collected and washed with H2O and then dried to provide the product. [0.942 g, 73%]
[Patent Reference: WO2012149280, page 73, ![]() (4.1 MB)]
(4.1 MB)]
Example 7
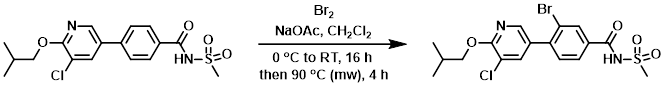
To an ice-cold solution of the SM (83.8 mg, 0.219 mmol) and NaOAc (35.9 mg, 0.438 mmol) in DCM (0.768 mL) was added dropwise a solution of Br2 (0.023 mL, 0.438 mmol) in DCM (0.768 mL) and the mixture was allowed to warm to RT over 16 h. To the mixture was added additional NaOAc (35.9 mg, 0.438 mmol) and Br2 (0.023 mL, 0.438 mmol) and the mixture was irradiated in a microwave reactor at 90 C for 4 h. The mixture was filtered through a frit and purified by RP-HPLC (0.1% TFA in ACN/H2O) to provide the product. [26.7 mg, 27%]
[Patent Reference: WO2015051043, page 88, ![]() (9.7 MB)]
(9.7 MB)]
Example 8

NBS (2.27 g, 12.75 mmol) was added to a solution of the SM (4.07 g, 12.75 mmol) in DCM (66 mL) at RT. The reaction mixture was stirred at RT for 18 h. The mixture was treated with sat aq NaHCO3 and stirred for 5 min. The org layer was separated, dried (MgSO4), and concentrated. The resulting material was purified by silica gel flash chromatography (5-20% EtOAc/heptane). The pure fractions were combined and concentrated. The residue was crystallized from EtOH, filtered, and dried under vacuum to provide the product as a pale beige powder. [3.30 g, 68%]
[Patent Reference: WO2016020526, page 35, ![]() (5.6 MB)]
(5.6 MB)]
