NBS
(N-Bromosuccinimide)
Other Names:
1-Bromo-2,5-pyrrolidinedione
General Information:
Structure:
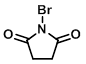
CAS Number: 128-08-5
Molecular Weight: 177.98 g/mol
Appearance: White solid
Melting Point: 175-180 C
N-Bromosuccinimide (NBS) is a reagent typically used in radical substitution and electrophilic addition reactions. In pure form it exists as a white solid but over time it will decompose to give off bromine which will give it a slight yellow/brown color.
Common Uses:
Reagent in SEAr (Electrophilic Aromatic Substitution) brominations
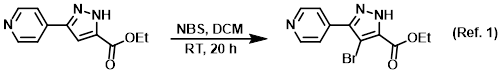
Procedure excerpt:
To a solution of the SM (34.7 g, 160 mmol) in DCM (464 mL) was added NBS (31.3 g, 176 mmol). The reaction mixture was stirred at RT for 20 h. The mixture was concentrated . . .
Reagent in brominations at benzylic positions

Procedure excerpt:
To a solution of the SM (261 mg, 1.00 mmol) in dry CCl4 (5 mL) in a 10 mL microwave vial was added benzoyl peroxide (12 mg, 0.05 mmol), followed by NBS (178 mg, 1.00 mmol). . . .
Reagent in the conversion of alcohols to bromides via Appel reactions
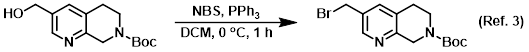
Procedure excerpt:
To a solution of the SM (0.261 g, 0.987 mmol) in DCM at 0 C was added NBS (0.211 g, 1.19 mmol), followed by PPh3 (0.311 g, 1.19 mmol). The reaction mixture . . .
References:
1) Patent Reference: WO2015144799, page 84, ![]() (18.8 MB)
(18.8 MB)
2) Patent Reference: WO2012112946, page 165, ![]() (11.2 MB)
(11.2 MB)
3) Patent Reference: WO2016014463, page 117, ![]() (6.7 MB)
(6.7 MB)
4) Wikipedia: N-Bromosuccinimide (link)
5) www.sigmaaldrich.com: N-Bromosuccinimide (link)
