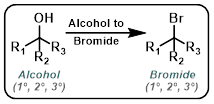
Alcohol to Bromide
![]()
Common Conditions:
PBr3
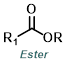
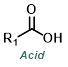
Phosphorus tribromide (PBr3) is possibly the most common reagent for the bromination of alcohols. The mechanism occurs by SN2 and results in the inversion of stereochemistry.[1]
![]()
Appel Reaction
The Appel reaction typically is described as PPh3 + CBr4. However, other bromine sources (ex. Br2 or NBS) are also used in conjunction with PPh3 to affect the same transformation. The mechanism is SN2 and results in the inversion of stereochemistry.[1]
![]()
SOBr2
Thionyl Bromide (SOBr2) is quite a bit more reactive than thionyl chloride (SOCl2), and is used much less frequently. SOBr2 can't be used with pyridine because it forms unreactive salts.
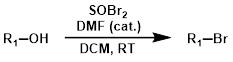
Reaction Map:
The reaction map is intended to provide insight into possible reactions one step before and after the title reaction. It also serves as an alternative way to navigate the website, and as a means of coming up with retrosynthetic ideas. Click on the reaction arrow to visit the page.
 |
||||
 |
 |
|||
 |
 |
 |
||
 |
 |
|||
 |
 |
|||
 |
References:
1) Carey, F. A.; Sundberg, R. J.; Advanced Organic Chemistry, Part B: Reactions and Synthesis
