Acid to Alcohol
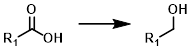
Common Conditions:
LiAlH4
Carboxylic acids are easily reduced by lithium aluminum hydride (LiAlH4) under mild conditions. One drawback is its lack of selectivity.[1]

BH3-THF
Borane (BH3) reagents are often able to selectively reduce carboxylic acids in the presence of esters. Decomposition of borane-tetrahydrofuran (BH3-THF) can generate H2 and tributyl borate. It is recommended that BH3-THF is used below 35 C (for safety). BH3-THF is typically only available in 1 M concentration.[2][3][4]

BH3-SMe2
Borane (BH3) reagents are often able to selectively reduce carboxylic acids in the presence of esters. Borane-dimethylsulfide (BH3-SMe2) is more stable than BH3-THF and is available in much higher concentrations (10 M). One drawback of BH3-SMe2 is its unpleasant odor.[2][3]
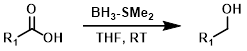
1) Activation 2) NaBH4
Carboxylic acids can be first activated (by a variety of different reagents), then reduced with sodium borohydride (NaBH4). This can be a useful alternative when substrates have functional groups that are incompatible with LiAlH4 or BH3.
Reaction Map:
The reaction map is intended to provide insight into possible reactions one step before and after the title reaction. It also serves as an alternative way to navigate the website, and as a means of coming up with retrosynthetic ideas. Click on the reaction arrow to visit the page.
 |
||||
 |
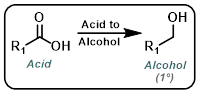 |
 |
||
 |
||||
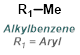 |
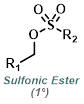 |
|||
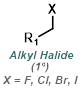 |
||||
References:
1) Smith, M. B.; March's Advanced Organic Chemistry, 7th Edition
2) Caron, S.; Practical Synthetic Organic Chemistry
3) Burke, S. D.; Danheiser, R. L.; Handbook of Reagents for Organic Synthesis, Oxidizing and Reducing Agents
4) Anderson, N. G.; Practical Process Research and Development, a Guide for Organic Chemists, 2nd Edition
