SNAr (Cl)
(Aliphatic Alcohols)
Examples:
Example 1
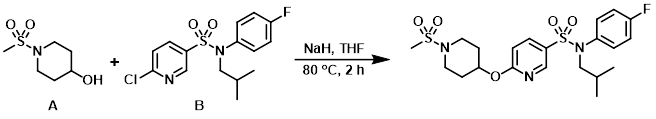
To a solution of the alcohol (A) (86 mg, 482 umol) in anhydrous THF (5 mL) at RT was added NaH (60% dispersion on mineral oil, 21 mg, 526 umol), and the mixture was stirred at RT for 15 min. The aryl chloride (B) (150 mg, 439 umol) was added and the reaction mixture was stirred at RT for 45 min, then 80 C for 2 h. H2O and sat aq NaHCO3 were added and the mixture was extracted with EtOAc, washed with brine, dried (Na2SO4), and concentrated. The resulting material was purified by silica gel column chromatography (0-25% EtOAc/cyclohexane) to provide the product. [137 mg]
[Patent Reference: WO2015177325, page 61, ![]() (4.3 MB)]
(4.3 MB)]
Example 2
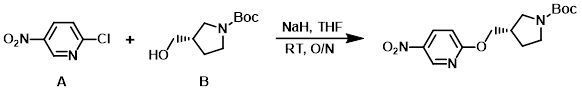
NaH (60%, 328 mg, 8.2 mmol) was suspended in THF (6 mL) at 0 C. To this mixture was added the alcohol (B) (1.269 g, 6.3 mmol) dissolved in THF (6 mL), and the aryl chloride (A) (1.00 g, 6.3 mmol) dissolved in THF (6 mL). The reaction mixture was stirred at RT overnight. The mixture was concentrated in vauco and the residue was partitioned between EtOAc and H2O. The org layer was washed with brine and dried by the use of a Whatman filter. The volatiles were removed in vacuo to provide the product which was used without further purification. [2.0 g, quant.]
[Patent Reference: WO2016012477, page 158, ![]() (8.1 MB)]
(8.1 MB)]
Example 3
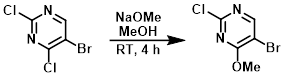
To a stirred solution of the SM (15 g, 65 mmol) in MeOH (150 mL) at RT under N2, was added NaOMe (3.91 g, 72 mmol) portionwise. The mixture was stirred at RT for 4 h and the solvent was removed in vacuo. The resulting solid was dissolved in CHCl3 (500 mL), washed with H2O, then brine. The org layer was dried (Na2SO4) and concentrated in vacuo to provide the product as a white solid. [13.5 g, 92%]
[Patent Reference: WO2010038081, page 86, ![]() (33.8 MB)]
(33.8 MB)]
Example 4
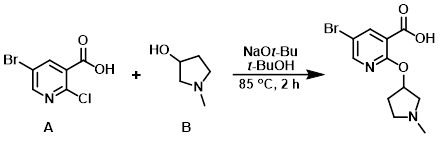
To the aryl chloride (A) (1.0 g, 4.21 mmol) and the alcohol (B) (1.77 g, 17.5 mmol) in t-BuOH (25 mL) was added NaOt-Bu (1.64 g, 16.9 mmol). The resulting mixture was heated at 85 C for 2 h, after which time the solvent was removed in vacuo and the reaction mixture was diluted with H2O. The aq layer was washed with EtOAc then carefully acidified with 1N HCl to pH 5. The mixture was concentrated to dryness. The resulting residue was dissolved in 1:1 MeOH/EtOAc, passed through a pad of celite, and concentrated to provide the crude product which was used in the next step without further purification. [2 g]
[Patent Reference: WO2010038081, page 202, ![]() (33.8 MB)]
(33.8 MB)]
Example 5
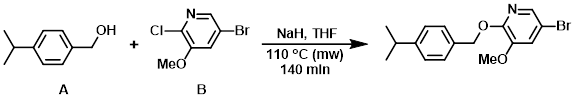
NaH (60%, 0.275 g, 6.87 mmol) was added to a stirred suspension of the alcohol (A) (0.967 g, 6.44 mmol) and the aryl chloride (B) (0.955 g, 4.29 mmol) in dry THF (16 mL) at 0 C under N2. The mixture was stirred under N2 at 0 C for 10 min. The vial was sealed and the reaction mixture was stirred at 110 C using a single mode microwave (Biotage Initiator EXP 60) with a power output ranging form 0 to 400 W for 140 min (fixed hold time). The mixture was quenched with H2O and extracted with EtOAc. The org layer was washed with brine, dried (MgSO4), and concentrated in vacuo. The solid residue was triturated in MeOH, filtered, and washed with MeOH to provide the product as a white solid. [0.785 g, 54%]
[Patent Reference: WO2015144799, page 102, ![]() (18.8 MB)]
(18.8 MB)]
Example 6
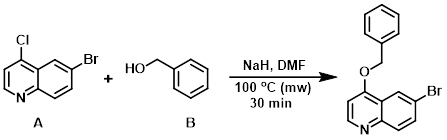
To a solution of NaH (60% in mineral oil) (1.75 equiv) in DMF (0.5 M) was added benzyl alcohol (B) (2.5 equiv) dropwise. After stirring 30 min, the aryl chloride (A) (1.0 equiv) was added and the solution was heated in a microwave reactor at 100 C for 30 min. Upon cooling, the solution was partitioned between EtOAc/H2O. The org layer was separated and washed with H2O (3x), brine, dried (MgSO4), concentrated, and triturated in hexanes to provide the product. [73%]
[Patent Reference: WO2010026121, page 59, ![]() (3.6 MB)]
(3.6 MB)]
Example 7
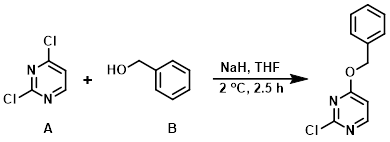
To a cooled (1-2 C) suspension of NaH (60% in mineral oil) (1.5 equiv) in THF (250 mL) was added benzyl alcohol (B) (1.0 equiv) dropwise. The mixture was stirred under N2 for 30 min then added in small portions (via syringe) over 1 h to a solution of the aryl chloride (A) (1.5 equiv) in THF also at 1-2 C. The resulting mixture (0.06 M) was stirred at <2 C for 2.5 h, then quenched with saturated aq NH4Cl and extracted with EtOAc. The org layer was separated, washed with brine, dried (Na2SO4), concentrated, and purified by silica gel column chromatography (hexane/DCM) to provide the product. [24%]
[Patent Reference: WO2010026121, page 55, ![]() (3.6 MB)]
(3.6 MB)]
