DCM
(Dichloromethane)
Other Names:
Methylene chloride
General Information:
Structure:
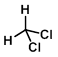
CAS Number: 75-09-2
Molecular Weight: 84.93 g/mol
Appearance: Colorless liquid
Chemical Formula: CH2Cl2
Melting Point: -95 C
Boiling Point: 40 C
Density: 1.33 g/mL
Common Uses:
Solvent for reactions
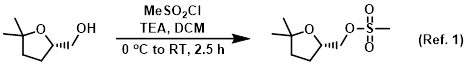
Procedure excerpt:
To a solution of the SM (300 mg, 2.30 mmol) in DCM (6 mL) at 0 C was added TEA (0.64 mL, 4.6 mmol) followed by MeSO2Cl (0.21 mL, 2.76 mmol). The reaction mixture was . . .
Solvent for extractions
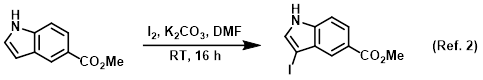
Procedure excerpt:
. . . The resulting mixture was extracted with DCM (2 x 200 mL). The combined extracts were dried (Na2SO4) and concentrated . . .
Solvent for silica gel chromatography
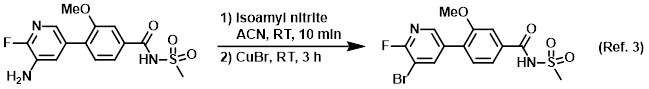
Procedure excerpt:
. . . The crude product was purified by silica gel column chromatography (0-10% MeOH/DCM) to provide the product . . .
Safety:
Dichloromethane (DCM) is considered toxic and may be a carcinogen. Prolonged contact with skin can dissolve fatty tissues and cause burns. The high volatility of DCM makes it a possible inhalation hazard. DCM is only considered to be slightly flammable.
References:
1) Patent Reference: WO2015129926, page 89, ![]() (21.5 MB)
(21.5 MB)
2) Patent Reference: WO2012129338, page 134, ![]() (12.0 MB)
(12.0 MB)
3) Patent Reference: WO2015051043, page 60, ![]() (9.7 MB)
(9.7 MB)
4) Wikipedia: Dichloromethane (link)
5) www.sigmaaldrich.com: Dichloromethane (link)
