Iodination
(SEAr)
Examples:
Example 1

To the SM (1.77 Kg, 8.58 mol) in anhydrous DMF (8 L) under N2 at 10 C was added NIS (1.93 Kg, 8.58 mol) in portions over 10 min. The reaction mixture was stirred at RT for 2 h, after which time it was cooled using an ice bath and quenched with sat aq Na2CO3 (5 L) and extracted with EtOAc (2 x 15 L). The combined organics were washed with sat aq Na2CO3 (2 x 5 L), H2O (3 x 2 L), dried (Na2SO4), and concentrated in vacuo. The resulting crude material was purified by silica gel column chromatography (25-40% EtOAc/hexanes) to provide the product. [1.47 Kg, 57% over 2 steps]
[Patent Reference: WO2012129344, page 123, ![]() (7.3 MB)]
(7.3 MB)]
Example 2

To a solution of the SM (400 mg, 2.467 mmol) in DMF (4.9 mL) was added Iodine (626 mg, 2.467 mmol) and KOH (346 mg, 6.17 mmol). The mixture was stirred at RT for 1 h, after which time it was poured into ice/H2O (30 mL) containing NaHSO3 (257 mg, 2.467 mmol). The reaction was acidified with 5M HCl resulting in a yellow ppt which was collected by filtration, washed with H2O, and dried overnight in a vacuum oven to provide the product. [516 mg, 72.6%]
[Patent Reference: WO2012129338, page 84, ![]() (12.0 MB)]
(12.0 MB)]
Example 3
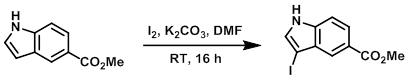
A brown solution of Iodine (3.98 g, 15.7 mmol) in DMF (4 mL) was added dropwise over 5 min to a yellow-brown suspension of the SM (2.5 g, 14.3 mmol) and K2CO3 (3.96 g, 28.7 mmol) in DMF (14 mL). The resulting mixture was stirred at RT for 16 h, after which time the reaction was added to mixture of ice (10 g), H2O (200 mL), and NaHSO3 (2.23 g, 21.4 mmol). The resulting mixture was extracted with DCM (2 x 200 mL). The combined extracts were dried (Na2SO4) and concentrated to provide the product as a brown solid. [3.87 g, 90%]
[Patent Reference: WO2012129338, page 134, ![]() (12.0 MB)]
(12.0 MB)]
Example 4
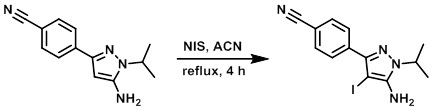
To a solution of the SM (1.45 g, 6.41 mmol) in ACN (26 mL) was added NIS (1.73 g, 7.69 mmol). The mixture was refluxed for 4 h and then allowed to stand at RT for 16 h. The reaction mixture was concentrated in vacuo and the crude residue partitioned between H2O (50 mL) and EtOAc (3 x 50 mL). The combined organics were dried (MgSO4) and concentrated to give a crude solid. The crude was purified by column chromatography (eluting with 30% EtOAc/pentane) to provide the product as a solid. [1.91 g, 85%]
[Patent Reference: WO2010032200, page 143, ![]() (6.2 MB)]
(6.2 MB)]
Example 5

A mixture of the SM (2.00 g, 8.09 mmol) and p-toluenesulfonic acid monohydrate (1.50 g, 7.89 mmol) in dry ACN (20 mL) was stirred for 10 min at RT, after which time the mixture was treated with NIS (1.82 g, 8.09 mmol) under vigorous stirring. After 2.5 h, the mixture was slowly poured into a cold solution of NaHCO3 (2.0 g) in 50 mL H2O. The resulting solid was filtered, washed with H2O (50 mL), and dried under vacuum overnight to provide the product as a brown solid. [2.35 g, 78%]
[Patent Reference: WO2012112946, page 206, ![]() (11.2 MB)]
(11.2 MB)]
Example 6
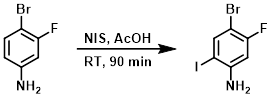
NIS (28.9 mmol) was added to a solution of the SM (5.00 g, 26.3 mmol) in AcOH (52.6 mL). The reaction mixture was stirred at RT for 90 min, after which time it was diluted with toluene (20 mL) and concentrated. The residue was dissolved in EtOAc and washed with 2N NaOH, brine, dried (Na2SO4), and concentrated. The resulting material was purified by silica gel chromatography (25 g silica gel loading column, 40 g Ultra SNAP column, 2-10% EtOAc/heptane) to provide the product. [7.23 g, 87%]
[Patent Reference: WO2014201173, page 375, ![]() (19.7 MB)]
(19.7 MB)]
Example 7

To a solution of the SM (500 mg, 2.94 mmol) in THF (15 mL) was added dropwise a solution of NIS (729 mg, 3.24 mmol) in THF (15 mL). The resulting suspension was stirred at RT for 2 h. The mixture was concentrated to dryness and dissolved in DCM. The solution was washed with sat aq Na2S2O5, dried (MgSO4), and concentrated. The crude material was purified by silica gel column chromatography to provide the product. [580 mg, 1.96 mmol]
[Patent Reference: WO2016020526, page 20, ![]() (5.6 MB)]
(5.6 MB)]
Example 8
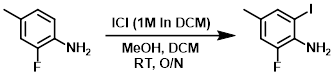
Iodine monochloride (1M in DCM, 8.9 mL, 8.9 mmol) was added dropwise over 20 min to a solution of the SM (0.5 mL, 4.45 mmol) in MeOH (5 mL) and DCM (10 mL) at RT. The reaction mixture was stirred at RT overnight. The mixture was cooled to 5 C and 1N NaOH was slowly added. The layers were separated and the org layer was washed with aq Na2SO3, H2O, dried (MgSO4), and concentrated. The residue was purified by silica gel flash chromatography (5% EtOAc/heptane) to provide the product as a colorless oil. [0.662 g, 95%]
[Patent Reference: WO2016020526, page 34, ![]() (5.6 MB)]
(5.6 MB)]
