Sodium Bisulfite
Other Names:
Sodium hydrogen sulfite
General Information:
Structure:
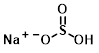
CAS Number: 7631-90-5
Molecular Weight: 104.061 g/mol
Appearance: White solid
Chemical Formula: NaHSO3
Melting Point: 150 C
Boiling Point: 315 C
Acidity (pKa): 6.97
Sodium bisulfite (NaHSO3) is a weakly acidic compound that is usually used as a mild reducing agent. Sodium bisulfite is typically used as an aqueous solution. A common application is in the workup of reactions involving bromine or iodine.
Common Uses:
Mild reducing agent for bromine or iodine reaction workups (ex. bromination or iodination)
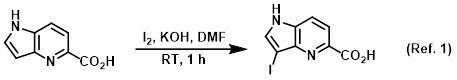
Procedure excerpt:
. . . The mixture was stirred at RT for 1 h, after which time it was poured into ice/H2O (30 mL) containing NaHSO3 (257 mg, 2.46 mmol). The reaction was . . .
Mild reducing agent for Jones oxidation workups
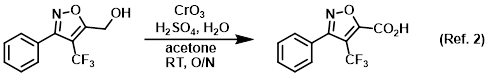
Procedure excerpt:
. . . The mixture was diluted with ether (600 mL) and washed with 2% aq NaHSO3 (5 x 100 mL). The layers were separated and the aq layer was . . .
References:
1) Patent Reference: WO2012129338, page 84, ![]() (12.0 MB)
(12.0 MB)
2) Patent Reference: WO2011017578, page 69, ![]() (8.3 MB)
(8.3 MB)
3) Wikipedia: Sodium bisulfite (link)
4) www.sigmaaldrich.com: Sodium bisulfite (link)
