Alcohol to Acid
(Jones Reagent)
Examples:
Example 1
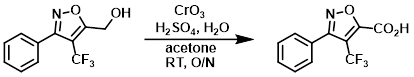
To an orange, homogeneous solution of CrO3 (12.4 g, 0.123 mol) in H2O (88.4 mL) at 0 C was added H2SO4 (10.8 mL) dropwise via addition funnel over 30 min, with stirring. The addition funnel was rinsed with H2O (1 mL) to give a 1.23 M solution of Jones Reagent. To a solution of the SM (5.24 g, 21.6 mmol) in acetone (75 mL) at RT (immersed in H2O bath) was added Jones Reagent (43.8 mL, 53.9 mmol) via addition funnel over 90 min. The dark reaction mixture was stirred at RT overnight. By HPLC, the reaction was 93% complete. Additional Jones Reagent (18 mL, 1.0 equiv) was added. After stirring another 6.5 h, HPLC indicated 97% completion. Isopropanol (6 mL) was added, and the mixture stirred for 90 min, resulting in a dark green precipitate. The mixture was diluted with ether (600 mL) and washed with 2% aq NaHSO3 (5 x 100 mL). The layers were separated and the aq layer was back-extracted with ether (2 x 100 mL). The combined organics were washed with H2O (100 mL), brine (100 mL), and dried (Na2SO4). The aq layer was back-extacted with ether (100 mL), and the resulting org layer was combined with previous organics. The organics were concentrated to provide the pdt as an off-white solid. The pdt was dissolved in DCM (200 mL), washed with 2% aq NaHSO3, brine, dried (Na2SO4), and concentrated to provide the pdt (96% purity) as a pale yellow solid [3.84 g, 69.3%]. Additional pdt remained in the NaHSO3 aq layer. The aq layer was saturated with NaCl, the pH adjusted to ~3.5, and extracted with ether (3 x 100 mL). The organics were dried (Na2SO4) and concentrated to provide the product (99% purity) as a white solid [1.12 g, 20.2%]. Total yield: [4.96 g, 89.5%]
[Patent Reference: WO2011017578, page 69, ![]() (8.3 MB)]
(8.3 MB)]
