Acetone
Other Names:
Propanone
Propan-2-one
2-Propanone
General Information:
Structure:
![]()
CAS Number: 67-64-1
Molecular Weight: 58.08 g/mol
Appearance: Colorless liquid
Melting Point: -94 C
Boiling Point: 56 C at 760 mmHg
Density: 0.791 g/mL at 25 C
Acetone is most commonly used in organic chemistry labs to clean glassware and make cooling baths. When combined with dry ice, acetone makes a cooling bath that has a temperature around -78 C. Acetone is miscible with water but does not form an azeotrope with water.
Common Uses:
Solvent in Jones oxidations
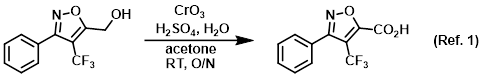
Procedure excerpt:
To a solution of the SM (5.24 g, 21.6 mmol) in acetone (75 mL) at RT (immersed in H2O bath) was added Jones Reagent (43.8 mL, 53.9 mmol) via addition funnel . . .
Reagent in reductive aminations
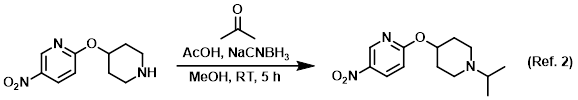
Procedure excerpt:
To a solution of 10% AcOH in MeOH was added the SM (1 equiv) and dry acetone (5 equiv). The solution was stirred at RT 1 h, after which time it was . . .
Reagent in Oppenauer oxidations
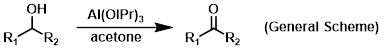
Safety:
Acetone is not considered to be a carcinogen, mutagenic chemical, or neurotoxin. In general, acetone is considered to be relatively safe compared to other solvents. For example, it is even used as the major component in many nail polish removers. However, care should still be taken to minimize exposure to acetone, as with all other solvents. The major danger of acetone is its high flammability. Acetone also has the ability to form highly explosive acetone peroxide in the presence of strong oxidizing reagents such as hydrogen peroxide.
References:
1) Patent Reference: WO2011017578, page 69, ![]() (8.3 MB)
(8.3 MB)
2) Patent Reference: WO2007084786, page 112, ![]() (9.4 MB)
(9.4 MB)
3) Wikipedia: Acetone (link)
4) www.sigmaaldrich.com: Acetone (link)
5) www.alfa.com: 22928 Acetone, HPLC Grade, 99.5% (link)
6) Kurti, L.; Czako, B.; Strategic Applications of Named Reactions in Organic Synthesis
