Hydrogen Peroxide
General Information:
Structure:
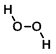
CAS Number: 7722-84-1
Molecular Weight: 34.01 g/mol
Appearance: Colorless liquid
Density: 1.11 g/mL (30% w/w aq.)
Hydrogen peroxide is the simplest peroxide and is considered a strong oxidizer. It exists as a colorless liquid and is miscible with H2O in all proportions. For laboratory purposes 30% Hydrogen peroxide (in H2O) is most common.
Common Uses:
Reagent for the oxidation of sulfides to sulfoxides or sulfones
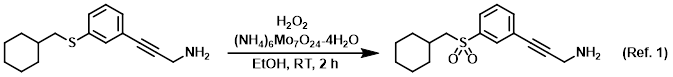
Procedure excerpt:
To a mixture of the SM (0.454 g, 1.59 mmol), (NH4)6Mo7O24-4H2O (0.585 g, 0.474 mmol), and absolute EtOH was added 30% H2O2 (1.6 mL, 15.7 mmol). The reaction mixture . . .
Reagent for the conversion of nitriles to amides (example below uses UHP)
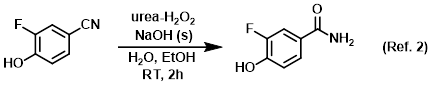
Procedure excerpt:
To a stirred solution of urea hydrogen peroxide (4.2 g, 43.8 mmol) in H2O (12 mL) was added NaOH (1.04 g, 25.5 mmol). The resulting mixture was cooled in an ice bath . . .
Reagent in the second step of the conversion or an aryl halide to an aryl-OH
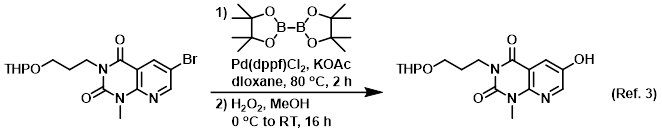
Procedure excerpt:
To a solution of the boronic ester intermediate (2.8 g, 6.3 mmol) in MeOH (100 mL) at 0 C was added H2O2 (30% in H2O, 1.4 mL, 10.6 mmol) dropwise over 5 min. The reaction . . .
Safety:
Hydrogen peroxide is a strong oxidizing agent. Hydrogen peroxide is well known to have the potential to cause explosive reactions. Handle with care.
References:
1) UK Pat App GB2463151A, page 120
2) Patent Reference: WO2012069948, page 63, ![]() (3.9 MB)
(3.9 MB)
3) Patent Reference: WO2016023830, page 98, ![]() (5.1 MB)
(5.1 MB)
4) www.h2o2.com
5) Wikipedia: Hydrogen peroxide (link)
6) www.sigmaaldrich.com: Hydrogen peroxide solution (link)
