Aryl-Halide to Aryl-OH
![]()
Common Conditions:
1) Borylation 2) H2O2
The conversion of aryl halides to aryl alcohols commonly involves a two-step process that first sees the conversion of the aryl halide to an organoborane. Hydrogen peroxide (H2O2) is typically used in the second step to cleave the C-B bond and provide the alcohol product.[1]
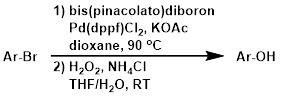
1) Borylation 2) NaBO3
The first step involves the conversion of the aryl halide to an organoborane. In the second step, sodium perborate (NaBO3) provides a easy to handle solid that hydrolyzes in the presence of H2O to give hydrogen peroxide (H2O2) and borate. NaBO3 is a convenient alternative to H2O2.[2]
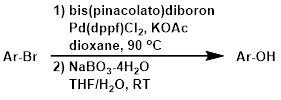
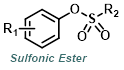
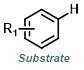
Reaction Map:
The reaction map is intended to provide insight into possible reactions one step before and after the title reaction. It also serves as an alternative way to navigate the website, and as a means of coming up with retrosynthetic ideas. Click on the reaction arrow to visit the page.
 |
||||
 |
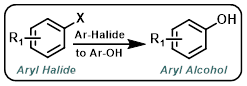 |
 |
||
 |
 |
|||
 |
References:
1) Burke, S. D.; Danheiser, R. L.; Handbook of Reagents for Organic Synthesis, Oxidizing and Reducing Agents
2) Wikipedia: Sodium perborate (link)
