Aryl-Halide to Aryl-OH
[1) Borylation 2) H2O2]
Examples:
Example 1
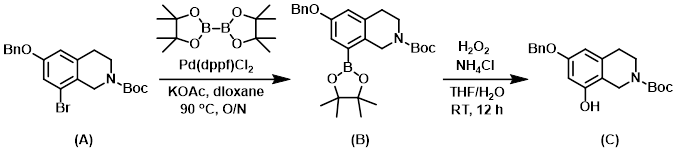
Step 1:
A solution of SM (A) (10 g, 24 mmol), bis(pinacolato)diboron (7.2 g, 29 mmol), KOAc (7.0 g, 71 mmol), and Pd(dppf)Cl2 (2 g) in dioxane (100 mL) was stirred at 90 C overnight. After filtration, the filtrate was concentrated in vacuo and the residue was purified by silica gel chromatography to provide intermediate (B). [5.4 g, 67%]
Step 2:
A solution of intermediate (B) (15 g, 32 mmol), NH4Cl (1.7 g, 120 mmol), and H2O2 (30%, 11 g, 97 mmol) in 1:1 THF/H2O (150 mL) was stirred at RT for 12 h. The mixture was quenched by the addition of an aq Na2S2O4 solution, and extracted with EtOAc. The org layer was dried and concentrated in vacuo. The residue was purified by silica gel flash chromatography to provide the product (C). [9.0 g, 79%]
[Patent Reference: WO2016014463, page 105, ![]() (6.7 MB)]
(6.7 MB)]
Example 2
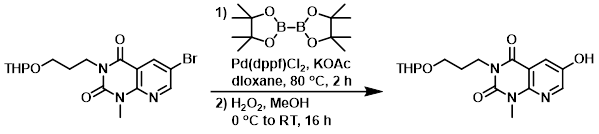
Step 1:
To a solution of the SM (2.5 g, 6.3 mmol) in dioxane (30 mL) was added bis(pinacolato)diboron (1.75 g, 6.9 mmol), Pd(dppf)Cl2 (0.46 g, 0.63 mmol), and KOAc (1.85 g, 18.9 mmol). The reaction mixture was heated at 80 C for 2 h. The mixture was cooled to RT, filtered through a pad of celite, and the pad was washed with EtOAc (2 x 50 mL). The filtrate was concentrated to provide the boronic ester intermediate as an oil. [2.80 g, 100%]
Step 2:
To a solution of the boronic ester intermediate (2.8 g, 6.3 mmol) in MeOH (100 mL) at 0 C was added H2O2 (30% in H2O, 1.4 mL, 10.6 mmol) dropwise over 5 min. The reaction mixture was allowed to warm slowly to RT and was stirred for 16 h. The mixture was quenched with aq 1M Na2SO3 (100 mL), stirred for 15 min, then extracted with EtOAc (3 x 75 mL). The combined organics were dried (Na2SO4) and concentrated to provide the product as a solid which was used without further purification. [2.1 g, 100%]
[Patent Reference: WO2016023830, page 98, ![]() (5.1 MB)]
(5.1 MB)]
