Ammonium Chloride
Other Names:
Salmiac
General Information:
Structure:
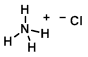
CAS Number: 12125-02-9
Molecular Weight: 53.49 g/mol
Appearance: White crystalline solid
Chemical Formula: NH4Cl
Melting Point: 338 C (sublimes)
Acidity (Pka): 9.24
Ammonium chloride (NH4Cl) is a solid that is often used as a source of ammonia (NH3) for reactions such as amide couplings. NH4Cl solutions are mildly acidic. Solutions of saturated aqueous ammonium chloride (sat aq NH4Cl) are very common in organic chemistry labs. Sat aq NH4Cl is typically used to quench reaction mixtures.
Common Uses:
Reagent in amide coupling reactions (serves as a source of NH3)
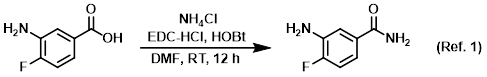
Procedure excerpt:
To a solution of the SM (1.0 g, 6.4 mmol) in DMF (5.0 mL) at 0 C was added EDC-HCl (1.2 g, 7.7 mmol), NH4Cl (1.4 g, 26.9 mmol), and HOBt (1.1 g, 8.3 mmol). . . .
Reagent in nitro reductions using iron
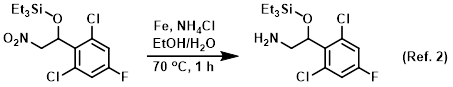
Procedure excerpt:
To a solution of the SM (15.0 g, 40.7 mmol) in 4:1 EtOH/H2O (60 mL) was added Fe powder (22.7 g, 407.6 mmol) and NH4Cl (21.8 g, 407.6 mmol). The reaction mixture was stirred . . .
Reagent in nitro reductions using zinc

Procedure excerpt:
To a solution of the SM (130 mg, 0.2 mmol) in dioxane/H2O (3:1, 2 mL) was added NH4Cl (97 mg, 1.5 mmol) and Zn dust (80 mg, 1.5 mmol) at 0 C. The reaction . . .
Saturated aqueous ammonium chloride is used to quench some basic reactions
Procedure excerpt:
…and the reaction mixture was stirred for 3 h. The mixture was quenched with sat aq NH4Cl (50 mL) and extracted with EtOAc . . .
Safety:
Mixing ammonia containing compounds (ex. NH3, NH4OH, or NH4Cl) and bleach (NaOCl) is very dangerous because it produces toxic by-pdts. People have died from mixing cleaning supplies that contain ammonia and bleach. Household cleaning products that contain ammonia are typically aqueous solutions (NH4OH).
References:
1) Patent Reference: WO2014149164, page 227, ![]() (23.7 MB)
(23.7 MB)
2) Patent Reference: WO2015129926, page 74, ![]() (21.5 MB)
(21.5 MB)
3) Patent Reference: WO2014149164, page 211, ![]() (23.7 MB)
(23.7 MB)
4) Patent Reference: WO2015129926, page 75, ![]() (21.5 MB)
(21.5 MB)
5) Wikipedia: Ammonium chloride (link)
6) www.sigmaaldrich.com: Ammonium chloride (link)
7) www.alfa.com: A15000 Ammonium chloride, 98% (link)
8) The Chlorine Institute, Sodium Hypochlorite Incompatibility Chart (link)
