Formylation
(Lithiation)
Examples:
Example 1
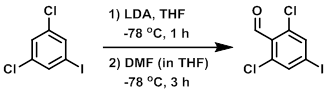
To a solution of the SM (4.0 g, 14.6 mmol) in THF (30 mL) at -78 C was added dropwise LDA (2.0M in THF/heptane/ethylbenzene, 9.6 mL, 16.9 mmol). The mixture was stirred at -78 C for 1 h. A solution of DMF (1.7 mL, 22.0 mmol) in THF (5 mL) was added slowly at - 78 C, and the reaction mixture was stirred for 3 h. The mixture was quenched with sat aq NH4Cl (50 mL) and extracted with EtOAc (2 x 30 mL). The combined organics were washed with H2O (50 mL), brine (50 mL), dried (Na2SO4), and concentrated. The resulting material was purified by silica gel column chromatography (20% EtOAc/hexane) to provide the product as a colorless oil. [1.4 g, 32%]
[Patent Reference: WO2015129926, page 75, ![]() (21.5 MB)]
(21.5 MB)]
Example 2
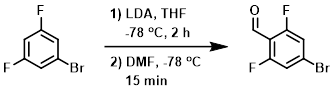
To a solution of the SM (210 g, 1.09 mol) in anhydrous THF (1.0 L) was added slowly LDA (2M in THF, 545 mL, 1.09 mol) under N2 at -78 C. The mixture was stirred at -78 C for 2 h. Anhydrous DMF (79.6 g, 1.09 mol) was added dropwise, and the reaction mixture was stirred at -78 C for an additional 15 min. A solution of 1:1 AcOH/EtOAc (300 mL) was added at -78 C to bring the mixture to pH = 4-5. The mixture was stirred at RT 15 min, concentrated, diluted with EtOAc (1.0 L), and washed with brine (2 x 600 mL). The org layer was dried (Na2SO4) and concentrated. The resulting material was recrystallized from n-hexane to provide the product as a light-yellow solid. [106 g, 44%]
[Patent Reference: WO2016011390, page 268, ![]() (20.2 MB)]
(20.2 MB)]
Example 3

To a mixture of the SM (5.5 g, 18.83 mmol) and tetramethylenediamine (2.95 mL, 18.83 mmol) in anhydrous THF was added dropwise LDA (1M in heptane, 38 mL, 37.67 mmol) at -20 C, and the mixture was stirred at this temperature for an additional 45 min. DMF (2.75 g, 37.67 mmol) was added dropwise over 15 min, and stirring was continued for 1 h. The mixture was diluted with sat aq NH4Cl (20 mL) and EtOAc (100 mL). The org layer was separated and the aq layer was further extracted with EtOAc (3 x 50 mL). The combined organics were washed with brine (100 mL), dried (Na2SO4), and concentrated. The resulting residue was purified by silica gel column chromatography (15% EtOAc/hexane) to provide the product as a yellow solid. [3.5 g, 58%]
[Patent Reference: WO2015088045, page 146, ![]() (10.3 MB)]
(10.3 MB)]
