Zinc
General Information:
Structure:
Zn
CAS Number: 7440-66-6
Molecular Weight: 65.39 g/mol
Appearance: Silver-gray solid
Zinc is a low cost reagent that is used in a wide variety of reduction reactions and in the making of organozinc reagents that allow the formation of new carbon-carbon bonds. Zinc sometimes requires activation before use.
Common Uses:
Reagent for reduction of nitro groups to amines
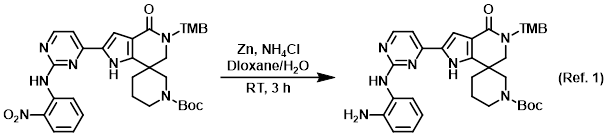
Procedure excerpt:
To a solution of the SM (130 mg, 0.2 mmol) in dioxane/H2O (3:1, 2 mL) was added NH4Cl (97 mg, 1.5 mmol) and Zn dust (80 mg, 1.5 mmol) at 0 C. The reaction mixture . . .
Reagent for the reduction of conjugated double bonds
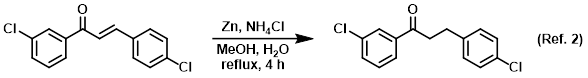
Procedure excerpt:
A mixture of the SM (1.38 g, 5 mmol), Zn dust (1.95 g, 30 mmol), NH4Cl (2.67 g, 50 mmol), MeOH (10 mL), and H2O (5 mL) were refluxed for 4 h. The mixture was cooled . . .
Reagent for the preparation of organozinc (Negishi) reagents
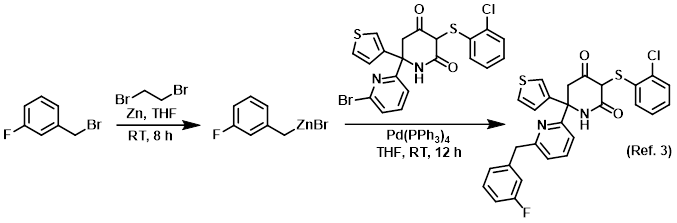
Safety:
Zinc is relatively non-toxic, although in large amounts it can be harmful.
References:
1) Patent Reference: WO2014149164, page 211, ![]() (23.7 MB)
(23.7 MB)
2) Patent Reference: WO2016023832, page 71, ![]() (3.2 MB)
(3.2 MB)
3) Patent Reference: WO2015140133, page 87, ![]() (11.7 MB)
(11.7 MB)
4) www.sigmaaldrich.com: Zinc - dust (link)
5) Burke, S. D.; Danheiser, R. L.; Handbook of Reagents for Organic Synthesis, Oxidizing and Reducing Agents
