Negishi
Examples:
Example 1
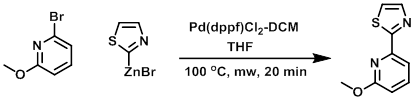
A mixture of the bromide (1.0 equiv), the organozinc bromide (0.5 M in THF, 2.0 equiv), and Pd(dppf)Cl2-DCM (0.2 equiv) in THF (0.1 M) was heated at 100 C for 20 min in a microwave reactor. Upon cooling, the solution was partitioned between EtOAc and sat aq Na2CO3. The org layer was separated and washed with brine, dried (MgSO4), concentrated, and purified by silica gel chromatography (20% EtOAc/hexane) to provide the product. [73%]
[Patent Reference: WO2010026121, page 53, ![]() (3.6 MB)]
(3.6 MB)]
Example 2
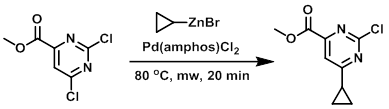
A 5 mL microwave vial containing the chloride (621 mg, 3.00 mmol) and Pd(amphos)Cl2 (53.1 mg, 0.075 mmol) was treated with the organozinc bromide (0.5 M in THF, 1.2 mL, 6.00 mmol) via syringe under argon. The solution was then irradiated in a microwave reactor at 80 C for 20 min. The resulting mixture was treated with EtOAc and 1N NaOH resulting in a white suspension as the zinc hydroxide precipitated out. The suspension was extracted with EtOAc (50 mL) a second time and the combined organics were washed with brine, dried (Na2SO4), concentrated, and purified by silica gel chromatography (eluting with 100% DCM) to provide the product as a clear, colorless, viscous oil. [254 mg, 40%]
[Patent Reference: WO2012129338, page 150, ![]() (12.0 MB)]
(12.0 MB)]
Example 3
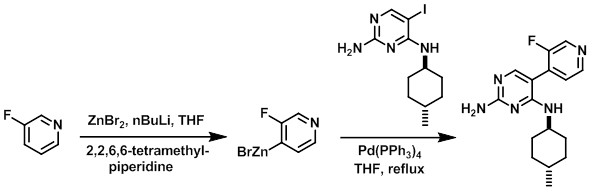
To a solution of 2,2,6,6-tetramethylpiperidine (997 mL, 5.87 mol) in dry THF (6 L) under N2 at 0 C was added n-BuLi (2.5 M in hexanes, 2.35 L, 5.87 mol) via an addition funnel over 30 min. Upon completion of the addition, the reaction mixture was stirred at 0 C for 1 h. The reaction mixture was cooled to -74 C and a solution of 3-fluoropyridine (561 g, 5.773 mmol) in dry THF (500 mL) was added over 15 min keeping the temp below -63 C. Upon completion of the addition, the reaction mixture was stirred at -74 C for 2 h. A solution of ZnBr2 (1.422 Kg, 6.32 mol) in dry THF (3 L) was then added dropwise over 35 min keeping the temp below -60 C. Upon completion of the addition, the reaction mixture was allowed to warm to RT and the Iodo compound (650 g, 1.95 mol) was added in one portion followed by Pd(PPh3)4 (113 g, 97.8 mmol). The reaction mixture was heated at reflux overnight, after which time the reaction mixture was cooled to RT and quenched with sat aq NaHCO3 (6 L) and extracted with EtOAc (2 x 10 L). The combined organics were washed with sat aq NaHCO3 (2 x 2.5 L) then brine (2.5 L) and concentrated in vacuo. The resulting crude was dissolved in 2N HCl (2.5 L) and washed with DCM (3 x 1.25 L). The aq phase was adjusted to pH 10-12 by the addition of aq 4N NaOH and extracted with DCM (3 x 1.5 L). The combined organics were washed with H2O (2 x 1.25 L), dried, and concentrated to provide the product. [540 g, 92%]
[Patent Reference: WO2012129344, page 124, ![]() (7.3 MB)]
(7.3 MB)]
