Sodium Carbonate
Other Names:
Carbonic acid disodium salt
General Information:
Structure:
![]()
CAS Number: 497-19-8
Molecular Weight: 105.99 g/mol
Appearance: White powder
Chemical Formula: Na2CO3
Sodium carbonate (Na2CO3) is a moderately strong base used commonly in organic chemistry. It is regularly employed as the base of choice for palladium catalyzed reactions such as Suzuki couplings. Sodium carbonate is also used for other reactions that require a base to neutralize acids that form during reactions. For example, alkylation reactions often employ sodium carbonate to mop up protons (acid) that forms during the reaction.
Common Uses:
Base for Suzuki reactions
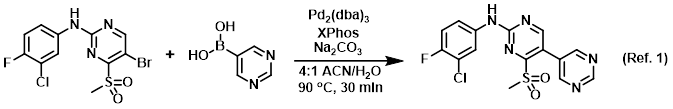
Procedure excerpt:
. . . the bromide (400 mg, 1.05 mmol), the boronic acid (208 mg, 1.68 mmol), Pd2(dba)3 (96 mg, 0.1 mmol), XPhos (148 mg, 0.3 mmol), and Na2CO3 (112 mg, 1.05 mmol) in ACN/H2O . . .
Base for substitution reactions
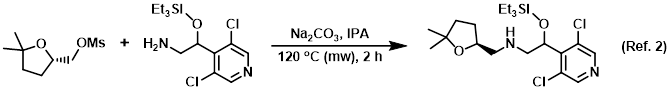
Procedure excerpt:
A mixture of the mesylate (140 mg, 0.67 mmol), the amine (216 mg, 0.67 mmol), Na2CO3 (710 mg, 6.7 mmol), and IPA (4 mL) were combined in a microwave vial. The vial . . .
Base for aq basic washes of org layer during workups

Procedure excerpt:
. . . The combined organics were washed with 1M HCl (10 mL), sat aq Na2CO3, brine, dried (Na2SO4), and concentrated . . .
Neutralization agent (ex. to neutralize/basify a mixture prior to extraction)
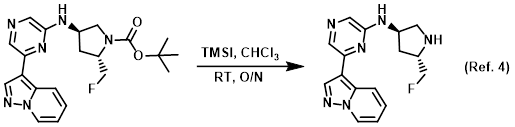
Procedure excerpt:
. . . The aq layer was then basified with 5% aq Na2CO3 to pH ~9, and was extracted with 3:1 CHCl3/IPA (3 x 30 mL). The combined organics were . . .
Safety:
Sodium carbonate (Na2CO3) is relatively non-toxic. For example, it is used for various purposes in the food and cosmetics industry. Sodium carbonate is a moderately strong base. It is a major irritant to skin and eyes. Store sodium carbonate separately from acids. Reactions with acids can be exothermic. Sodium carbonate is incompatible with aluminum.
References:
1) Patent Reference: WO2010038081, page 109, ![]() (33.8 MB)
(33.8 MB)
2) Patent Reference: WO2015129926, page 89, ![]() (21.5 MB)
(21.5 MB)
3) Patent Reference: WO2016011390, page 112, ![]() (20.2 MB)
(20.2 MB)
4) Patent Reference: WO2010016005, page 125, ![]() (11.3 MB)
(11.3 MB)
5) Wikipedia: Sodium carbonate (link)
6) www.sigmaaldrich.com: Sodium carbonate (link)
