Substitution (Mesylate)
(Aliphatic Amines)
Examples:
Example 1
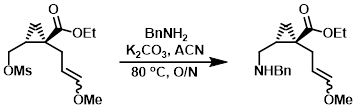
To a solution of the SM (3.8 g, 13.7 mmol) in ACN (30 mL) was added benzylamine (2.2 mL, 20.5 mmol), followed by K2CO3 (5.7 g, 41.0 mmol). The reaction mixture was heated at 80 C overnight, after which time it was cooled to RT. A precipitate formed which was removed by filtration and the filter pad was rinsed thoroughly with ACN. The filtrate was concentrated in vacuo and the residue was purified by silica gel flash chromatography to provide the product. [2.6 g, 66%]
[Patent Reference: WO2016014463, page 63, ![]() (6.7 MB)]
(6.7 MB)]
Example 2
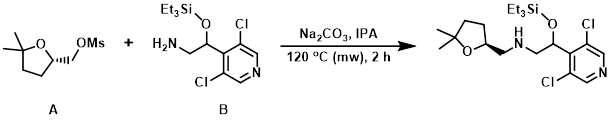
A mixture of the mesylate (A) (140 mg, 0.67 mmol), the amine (B) (216 mg, 0.67 mmol), Na2CO3 (710 mg, 6.7 mmol), and IPA (4 mL) were combined in a microwave vial. The vial was capped and the reaction mixture was heated in a microwave reactor at 120 C for 2 h. The mixture was cooled to RT, quenched with H2O (15 mL), and extracted with DCM (2 x 25 mL). The combined organics were washed with brine (20 mL), dried (Na2SO4), and concentrated. The resulting material was purified by silica gel column chromatography (2% MeOH/DCM) to provide the product as a colorless gum. [40 mg, 14%]
[Patent Reference: WO2015129926, page 89, ![]() (21.5 MB)]
(21.5 MB)]
