IPA
(Isopropanol)
Other Names:
Isopropyl alcohol
2-Propanol
Propan-2-ol
sec-Propyl alcohol
General Information:
Structure:
![]()
CAS Number: 67-63-0
Molecular Weight: 60.10 g/mol
Appearance: Colorless liquid
Melting Point: -89 C
Boiling Point: 82 C
Density: 0.785 g/mL at 25 C
Isopropanol is used occasionally in organic chemistry as a solvent. A common use would be in reactions that require a higher boiling protic solvent. Isopropanol is also commonly used as the solvent of choice for the cold finger of a rotovap.
Common Uses:
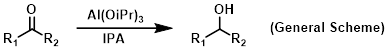
Reagent in Meerwein-Poondorf-Verley reactions
Reagent for Mitsunobu reactions
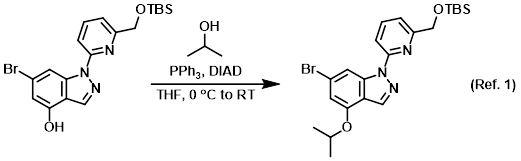
Procedure excerpt:
PPh3 (0.55 g, 2.1 mmol) was added to a solution of the SM (0.65 g, 1.5 mmol) and IPA (0.16 mL, 2.1 mmol) in THF (7.6 mL). The mixture was cooled in an ice-H2O bath, then . . .
Solvent for reactions
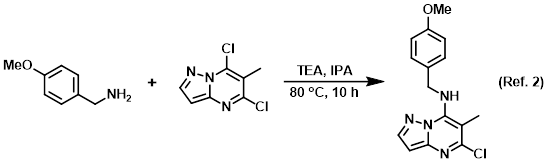
Procedure excerpt:
To a solution of the aryl chloride (1.0 g, 5.0 mmol) in IPA (50 mL) at RT was added the amine (817 mg, 6.0 mmol) and TEA (989 mg, 10.0 mmol). The reaction mixture . . .
Solvent/co-solvent for extractions

Procedure excerpt:
. . . The aq layer was then basified with 5% aq Na2CO3 to pH ~9, and was extracted with 3:1 CHCl3/IPA (3 x 30 mL). The combined organics . . .
Safety:
Isopropanol is toxic, but less toxic than methanol. Isopropanol is a flammable liquid.
References:
1) Patent Reference: WO2016011390, page 469, ![]() (20.2 MB)
(20.2 MB)
2) Patent Reference: WO2014149164, page 296, ![]() (23.7 MB)
(23.7 MB)
3) Patent Reference: WO2010016005, page 125, ![]() (11.3 MB)
(11.3 MB)
1) Wikipedia: Isopropyl alcohol (link)
2) www.sigmaaldrich.com: 2-Propanol (link)
