Triphenylphosphine
Other Names:
Triphenylphosphane
Phosphorustriphenyl
General Information:
Structure:
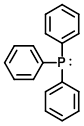
CAS Number: 603-35-0
Molecular Weight: 262.29 g/mol
Appearance: White solid
Triphenylphosphine is often used as a reducing agent, leading to the formation of triphenylphosphine oxide. The reaction is driven by the formation of the thermodynamically favored P=O bond of triphenylphosphine oxide. Triphenylphosphine oxide is a side-product that can be difficult to separate from desired product. Triphenylphosphine oxide is often removed through chromatography. When exposed to air, triphenylphosphine slowly oxidizes to triphenylphosphine oxide.
Common Uses:
Reagent in Mitsunobu reactions
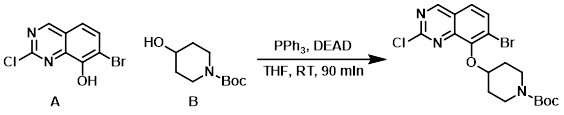
Procedure excerpt:
To a solution of A (400 mg, 1.54 mmol), B (621 mg, 3.09 mmol), and PPh3 (610 mg, 2.32 mmol) in THF (5.5 mL) at RT was added DEAD (404 mg, 2.32 mmol) . . .
Reagent in Appel reactions
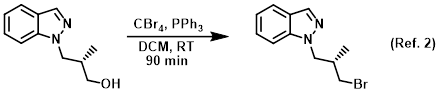
Procedure excerpt:
The SM (6.8 g, 0.036 mol) was taken up in DCM (275 mL) and treated with PPh3 (10.36 g) and CBr4 (13.1 g). The reaction mixture was stirred at RT for 90 min . . .
Reagent in Staudinger reactions
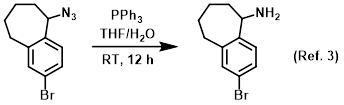
Procedure excerpt:
To a mixture of the SM (375 mg, 1.4 mmol) in THF (5 mL) and H2O (0.5 mL) was added PPh3 (741 mg, 2.8 mmol). The reaction mixture was stirred at RT for 12 h. . . .
Reagent for phosphonium salt preparation (usually used later in Wittig reactions)
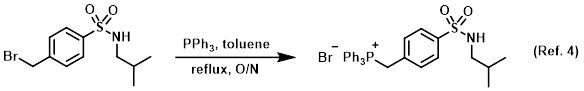
Procedure excerpt:
A mixture of the SM (1.08 g, 3.53 mmol) and PPh3 (1.39 g, 5.29 mmol) in toluene (20 mL) was stirred at reflux for 18 h. The mixture was cooled and the precipitate was . . .
Safety:
For acute exposure triphenylphosphine is considered an irritant, for chronic exposure it poses neurological risks. Triphenylphosphine is incompatible with strong oxidizing agents.
References:
1) Patent Reference: WO2007117607, page 312, ![]() (12.9 MB)
(12.9 MB)
2) Patent Reference: WO2014152144, page 56, ![]() (4.6 MB)
(4.6 MB)
3) Patent Reference: WO2015089337, page 185, ![]() (17.5 MB)
(17.5 MB)
4) Patent Reference: WO2015177325, page 77, ![]() (4.3 MB)
(4.3 MB)
5) Burke, S. D.; Danheiser, R. L.; Handbook of Reagents for Organic Synthesis, Oxidizing and Reducing Agents
6) Wikipedia: Triphenylphosphine (link)
7) www.sigmaaldrich.com: Triphenylphosphine (link)
