Staudinger
(PPh3 + H2O)
Examples
Example 1
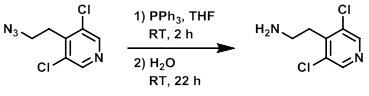
To a solution of the SM (2.4 g, 11.1 mmol) in THF (25 mL) at 0 C was added PPh3 (2.9 g, 22.1 mmol). The mixture was stirred at RT for 2 h. H2O (2.5 mL) was added and the reaction mixture was stirred at RT for 22 h. The mixture was quenched with 2M HCl (10 mL) and washed with EtOAc (3x). The aq layer was basified with 2M NaOH to pH 12, then extracted with EtOAc. The combined organics were washed with brine (2x), dried (MgSO4), concentrated, and dried under high vac to provide the product as a white solid. [1.9 g, 90%]
[Patent Reference: WO2015129926, page 146, ![]() (21.5 MB)]
(21.5 MB)]
Example 2
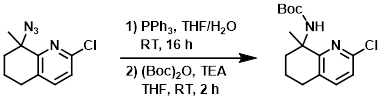
To a mixture of the SM (120 mg, 0.54 mmol) in THF/H2O (10 mL/0.5 mL) was added PPh3 (425 mg, 1.6 mmol). The reaction mixture was stirred at RT for 16 h. To the mixture was then added THF (5 mL), (Boc)2O (177 mg, 0.81 mmol), and TEA (164 mg, 1.62 mmol). The mixture was stirred at RT for 2 h. After concentration, the residue was purified by silica gel column chromatography (10:1 PE/EtOAc) to provide the product as a colorless oil. [300 mg, crude]
[Patent Reference: WO2016011390, page 357, ![]() (20.2 MB)]
(20.2 MB)]
Example 3
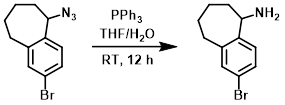
To a mixture of the SM (375 mg, 1.4 mmol) in THF (5 mL) and H2O (0.5 mL) was added PPh3 (741 mg, 2.8 mmol). The reaction mixture was stirred at RT for 12 h. The mixture was acidified to pH = 1 with 1N HCl and extracted with EtOAc (100 mL). The aq layer was separated and basified to pH = 10 with 1N NaOH. The resulting precipitate was collected and dried to provide the product as a white solid. [360 mg, 100%]
[Patent Reference: WO2015089337, page 185, ![]() (17.5 MB)]
(17.5 MB)]
