Carbon Tetrabromide
Other Names:
Tetrabromomethane
General Information:
Structure:
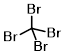
CAS Number: 558-13-4
Molecular Weight: 331.63 g/mol
Appearance: Colorless to yellow-brown crystals
Chemical Formula: CBr4
Melting Point: 88-90 C
Boiling Point: 190 C
Density: 3.42 g/mL
Carbon tetrabromide (CBr4) is most commonly used as a reagent for converting alcohols to bromides in Appel reactions. Carbon tetrabromide is generally not used as a solvent, whereas its chloro analogue, carbon tetrachloride (CCl4), sees frequent use as a solvent.
Common Uses:
Reagent for converting alcohols to bromides via the Appel reaction
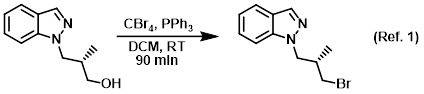
Procedure excerpt:
The SM (6.8 g, 0.036 mol) was taken up in DCM (275 mL) and treated with PPh3 (10.36 g) and CBr4 (13.1 g). The reaction mixture was stirred at RT for 90 min, after which time . . .
References:
1) Patent Reference: WO2014152144, page 56, ![]() (4.6 MB)
(4.6 MB)
2) Wikipedia: Tetrabromomethane (link)
3) www.sigmaaldrich.com: Tetrabromomethane (link)
4) Coates, R. M.; Denmark, S. E.; Handbook of Reagents for Organic Synthesis, Reagents, Auxiliaries, and Catalysts for C-C Bond Formation
