Carbon Tetrachloride
Other Names:
Tetrachloromethane
General Information:
Structure:
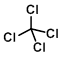
CAS Number: 56-23-5
Molecular Weight: 153.82 g/mol
Appearance: Colorless liquid
Chemical Formula: CCl4
Melting Point: -23 C
Boiling Point: 76-77 C
Density: 1.594 g/mL
Due to its symmetrical structure, carbon tetrachloride (CCl4) is non-polar. Its non-polar nature makes it a suitable solvent for dissolving non-polar compounds. Carbon tetrachloride is convenient as a solvent for NMR because it contains no protons, however, its poor dissolving power limits its usefulness.
Common Uses:
Solvent in benzylic brominations
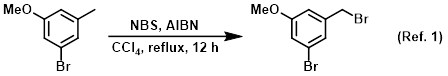
Procedure excerpt:
A mixture of the SM (100 g, 497 mmol), NBS (88.5 g, 497 mmol), and AIBN (10 g, 50 mmol) in CCl4 (700 mL) was stirred at reflux for 12 h. The mixture was cooled . . .
Safety:
Carbon tetrachloride is toxic to the central nervous system. It is not flammable, and actually at one time was used in fire extinguishers.
References:
1) Patent Reference: WO2016014463, page 101, ![]() (6.7 MB)
(6.7 MB)
2) Wikipedia: Carbon tetrachloride (link)
3) www.sigmaaldrich.com: Carbon tetrachloride (link)
