Bromination
(Benzylic Bromination)
Examples:
Example 1
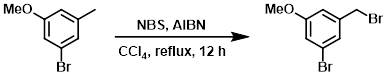
A mixture of the SM (100 g, 497 mmol), NBS (88.5 g, 497 mmol), and AIBN (10 g, 50 mmol) in CCl4 (700 mL) was stirred at reflux for 12 h. The mixture was cooled to RT, diluted with H2O, and extracted with EtOAc. The org layer was separated, dried (Na2SO4), and concentrated. The residue was purified by silica gel flash chromatography to provide the product. [48 g, 42%]
[Patent Reference: WO2016014463, page 101, ![]() (6.7 MB)]
(6.7 MB)]
Example 2
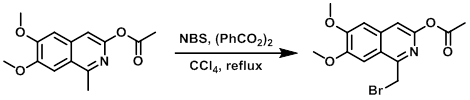
To a solution of the SM (261 mg, 1.00 mmol) in dry CCl4 (5 mL) in a 10 mL microwave vial was added benzoyl peroxide (12 mg, 0.05 mmol), followed by NBS (178 mg, 1.00 mmol). The resulting mixture was irradiated in a microwave reactor at 130 C for 15 min. The reaction mixture was diluted with DCM (20 mL), washed with brine (5 mL), dried (Na2SO4), concentrated, and purified by silica gel column chromatography (0-25% EtOAc/cyclohexane) to provide the product as a yellow solid. [140 mg, 41%]
[Patent Reference: WO2012112946, page 165, ![]() (11.2 MB)]
(11.2 MB)]
Example 3
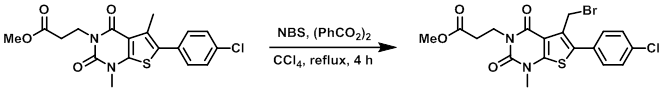
To a solution of the SM (25 mg, 0.0636 mmol) in CCl4 (1 mL) was added NBS (11.3 mg, 0.0636 mmol) followed by benzoyl peroxide (2 mg). The reaction was stirred at reflux for 4 h. The mixture was cooled to RT and diluted with DCM (10 mL). The mixture was washed with aq NaHCO3 (5 mL), brine (10 mL), dried (Na2SO4), and concentrated to provide the product which was used without further purification. [30 mg, 100%]
[Patent Reference: WO2016023832, page 66, ![]() (3.2 MB)]
(3.2 MB)]
