Mitsunobu
(Aromatic Alcohols)
Examples:
Example 1
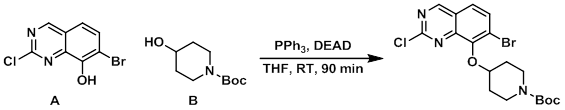
To a solution of A (400 mg, 1.54 mmol), B (621 mg, 3.09 mmol), and PPh3 (610 mg, 2.32 mmol) in THF (5.5 mL) at RT was added DEAD (404 mg, 2.32 mmol) in THF (0.5 mL). After 90 min, silica gel was added and the mixture was concentrated and purified on a flash column (25% EtOAc/hexane) to provide the product. [545 mg, 80%]
[Patent Reference: WO2007117607, page 312, ![]() (12.9 MB)]
(12.9 MB)]
Example 2
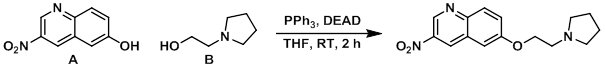
To a mixture of A (148 mg, 0.78 mmol) in THF (18 mL) was added B (0.091 mL, 0.78 mmol) and PPh3 (306 mg, 1.17 mmol). Lastly, DEAD (0.184 mL, 1.17 mmol) was added and the reaction mixture was stirred at RT for 2 h. Upon completion, the reaction mixture was concentrated and purified by silica gel flash chromatography to provide the product. [134 mg, 60%]
[Patent Reference: WO2007084786, page 115, ![]() (9.4 MB)]
(9.4 MB)]
Example 3
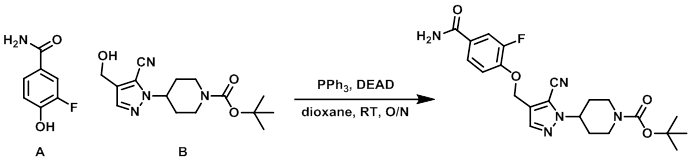
To a solution of B (200 mg, 0.65 mmol), A (100 mg, 0.64 mmol), and PPh3 (188 mg, 0.72 mmol) in dioxane (3 mL) was added dropwise DEAD (0.11 mL, 0.69 mmol). The resulting mixture was stirred at RT overnight, after which time it was concentrated in vacuo. The resulting material was purified by flash chromatography (eluting with 30-70% EtOAc/heptane) to provide the product as a white solid. [215 mg]
[Patent Reference: WO2012069948, page 90, ![]() (3.9 MB)]
(3.9 MB)]
Example 4

To a suspension of A (5.12 g, 34.1 mmol), B (5.00 g, 22.7 mmol), PPh3 supp. (8.94 g, 34.1 mmol) in dry DCM (150 mL) was added DtBAD (7.85 g, 34.1 mmol). The reaction mixture was stirred at RT for 18 h, after which time it was filtered through a glass frit and washed with EtOAc. The filtrate was concentrated in vacuo to give 27 g of crude material (yellow oil) that was purified by silica gel chromatography (150 g silica, 10% EtOAc/heptane) to provide the product as a white gum. [8.00 g, quant.]
[Patent Reference: WO2015144799, page 85, ![]() (18.8 MB)]
(18.8 MB)]
Example 5
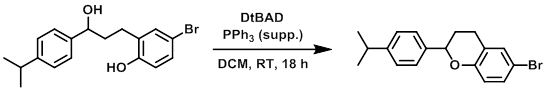
DtBAD (5.93 g, 25.8 mmol) was added to a stirred solution of the SM (6.00 g, 17.2 mmol) and PPh3 supp. (8.05 g, 25.8 mmol) in DCM (70 mL) at RT. The reaction mixture was stirred at RT for 18 h. H2O was added to the mixture and the solution was extracted with DCM. The org layer was dried (MgSO4) and concentrated. The resulting material was purified by Prep LC (80 g silica, 5% EtOAc/heptane) to provide the product as a white solid. [3.14 g, 55%]
[Patent Reference: WO2015144799, page 304, ![]() (18.8 MB)]
(18.8 MB)]
Example 6

A suspension of A (22.2 g, 216.9 mmol), B (22.0 g, 144.6 mmol), PPh3 (3.8 g, 14.5 mmol), and DEAD (28.0 g, 159.1 mmol) in THF (150 mL) was refluxed for 8 h. After cooling to RT, the reaction mixture was diluted with H2O (60 mL) and EtOAc (120 mL). The org layer was concentrated and the resulting crude residue was purified by silica gel column chromatography (3:1 petroleum ether/EtOAc) to provide the product as a brown oil. [18.1 g, 53%]
[Patent Reference: WO2015140133, page 117, ![]() (11.7 MB)]
(11.7 MB)]
Example 7
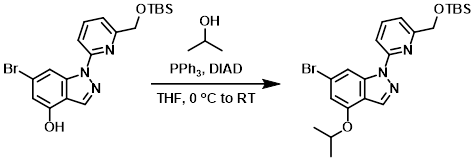
PPh3 (0.55 g, 2.1 mmol) was added to a solution of the SM (0.65 g, 1.5 mmol) and IPA (0.16 mL, 2.1 mmol) in THF (7.6 mL). The mixture was cooled in an ice-H2O bath, then treated slowly with DIAD (0.41 mL, 2.1 mmol). The mixture was allowed to reach RT while stirring over the weekend. The mixture was diluted with EtOAc then washed with H2O, brine, dried (MgSO4), and concentrated. The resulting material was purified by silica gel flash chromatography (24 g silica gel, 0-40% EtOAc/heptane) to provide the product. [0.608 g, 86%]
[Patent Reference: WO2016011390, page 469, ![]() (20.2 MB)]
(20.2 MB)]
Example 8
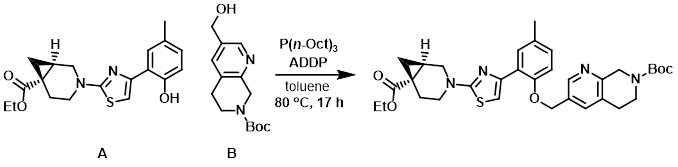
A solution of A (0.0875 g, 0.244 mmol) and crude B (0.16 g, 0.61 mmol) in toluene (4.0 mL) was sparged with N2 for 30 min. To this mixture was added ADDP (0.192 g, 0.761 mmol) and the mixture was again sparged with N2 and stirred an additional 10 min. Trioctylphosphine (0.40 mL, 0.81 mmol) was added and the reaction mixture was stirred at 80 C for about 17 h. The mixture was cooled to RT and concentrated in vacuo. The crude material was purified by silica gel flash chromatography to provide the product. [0.142 g, 96%]
[Patent Reference: WO2016014463, page 168, ![]() (6.7 MB)]
(6.7 MB)]
Example 9

To a pressure vial was added A (0.090 mL, 1.04 mmol), B (0.100 g, 0.520 mmol), and ADDP (0.394 g, 1.56 mmol). Then, anhydrous toluene (5 mL) and P(n-Bu)3 (0.390 mL, 1.56 mmol) were added, and the reaction mixture was stirred at 140 C in a microwave reactor for 15 min. The mixture was quenched with MeOH (1 mL), diluted with EtOAc (50 mL), celite was added, and the solvent was removed in vacuo. The material was purified by flash chromatography (solid loading on celite) to provide the product as a white solid. [0.124 g, 88%]
[Patent Reference: WO2016010950, page 201, ![]() (18.8 MB)]
(18.8 MB)]
