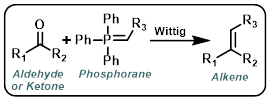
Wittig
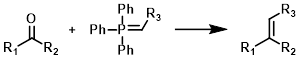
Common Conditions:
Phosphonium Salt Preparation
Prior to performing a Wittig reaction it is often necessary to prepare a phosphonium salt. This usually involves simply reacting PPh3 with an alkyl halide.[1]

Wittig Reagent (in situ)
Wittig reagents (also called phosphorus ylids or phosphoranes) are typically formed in situ by the treatment of a phosphonium salt with strong base (ex. t-BuOK, n-BuLi, or NaH). The aldehyde or ketone it often added after Wittig reagent formation has had time to complete.[1][2]
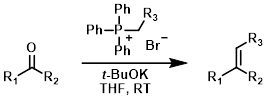
Wittig Reagent (stable)
Wittig reagents where R3 is capable of stabilizing the negative charge from the carbanion through resonance (ex. CO2R) are much more stable (often commercially available). These reagents are less reactive. They readily react with aldehydes but often fail to react with ketones.[1][2]
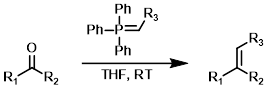
One-Pot Wittig
Alpha-halo carbonyl compounds and benzylic halides can form Wittig reagents in the presence of moderate/weak bases. These conditions are compatible with the presence of aldehydes or ketones and therefore can be conducted in a one-pot manner.[3]
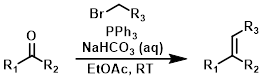
Aza-Wittig
The reaction of iminophosphoranes with aldehydes or ketones gives the imine product. The iminophosphoranes are typically formed from the reaction of the corresponding azide (R3-N3) with triphenylphosphine (PPh3).
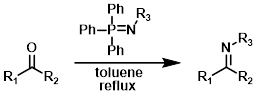
Reaction Map:
The reaction map is intended to provide insight into possible reactions one step before and after the title reaction. It also serves as an alternative way to navigate the website, and as a means of coming up with retrosynthetic ideas. Click on the reaction arrow to visit the page.
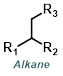
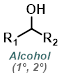
| For Aldehyde: | ||||
 |
||||
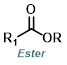 |
 |
|||
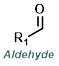 |
||||
 |
 |
|||
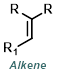 |
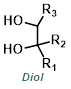 |
|||
| For Ketone: | ||||
 |
 |
|||
 |
 |
|||
 |
||||
 |
||||
Related Books:
References:
1) Smith, M. B.; March's Advanced Organic Chemistry, 7th Edition
2) Wikipedia: Wittig Reaction (Link)
2) Wang, J.; Stereoselective Alkene Synthesis
