Alcohol to Ketone

Common Conditions:
Dess-Martin Periodinane
The Dess-Martin periodinane conditions are considered to be relatively mild. Reactions are typically run at RT for a couple of hours. The most common solvent is DCM.[1]
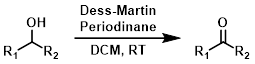
Swern Oxidation
The conditions employed for Swern oxidations typically start at very low temps (-78 C), and then stir at RT for several hours. The process produces foul smelling dimethyl sulfide gas.[1]
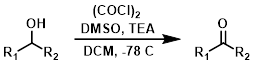
MnO2
Manganese dioxide (MnO2) is a mild reagent that is usually used for the oxidation of allylic or benzylic alcohols. The reaction is commonly done in DCM.
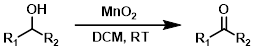
PCC
The use of pyridinium chlorochromate (PCC) has declined over time due to its toxicity. The reaction is typically done in DCM.[1]
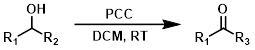
Reaction Map:
The reaction map is intended to provide insight into possible reactions one step before and after the title reaction. It also serves as an alternative way to navigate the website, and as a means of coming up with retrosynthetic ideas. Click on the reaction arrow to visit the page.
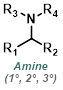 |
|||||
 |
|||||
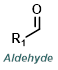 |
|||||
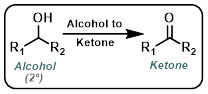 |
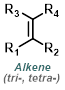 |
||||
 |
|||||
 |
|||||
 |
|||||
References:
1) Tojo, G.; Fernandez, M.; Oxidation of Alcohols to Aldehydes and Ketones
