Manganese Dioxide
Other Names:
Manganese(IV) oxide
General Information:
Structure:
MnO2
CAS Number: 1313-13-9
Molecular Weight: 86.94 g/mol
Appearance: Black solid
Manganese(IV) oxide (MnO2) is a reagent typically used for the oxidation of allylic or benzylic alcohols to their corresponding aldehyde or ketone.
Common Uses:
Reagent for the oxidation of alcohols to aldehydes
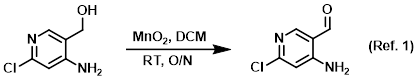
Procedure excerpt:
To a solution of the SM (10 g, 63.3 mmol) in DCM (150 mL) was added activated MnO2 (38 g, 443 mmol). The reaction mixture was stirred at RT overnight. The solids were . . .
Reagent for the oxidation of alcohols to ketones
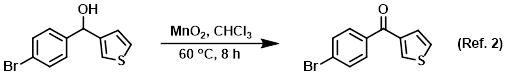
Procedure excerpt:
To a solution of the SM (100 g, 371.5 mmol) in CHCl3 (200 mL) was added MnO2 (322.9 g, 3.715 mol). The reaction mixture was stirred at 60 C for 12 h. After cooling . . .
References:
1) Patent Reference: WO2013134298, page 40 , ![]() (4.1 MB)
(4.1 MB)
2) Patent Reference: WO2015140133, page 90, ![]() (11.7 MB)
(11.7 MB)
3) Wikipedia: Manganese dioxide (link)
4) www.sigmaaldrich.com: Manganese(IV) oxide (link)
5) Burke, S. D.; Danheiser, R. L.; Handbook of Reagents for Organic Synthesis, Oxidizing and Reducing Reagents
