Alcohol to Ketone
(Dess-Martin Periodinane)
Examples:
Example 1
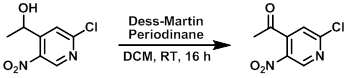
To a solution of the SM (1.0 equiv) in DCM (0.1 M) was added Dess-Martin periodinane (1.8 equiv). The mixture was stirred at RT for 16 h, after which time it was poured into EtOAc (800 mL), washed with 1:1 10% Na2S2O3/sat aq NaHCO3 (4x), then sat aq NaCl. The org layer was dried (MgSO4) and concentrated in vacuo to provide to product as a yellow solid. [96%]
[Patent Reference: WO2010026121, page 33, ![]() (3.6 MB)]
(3.6 MB)]
Example 2
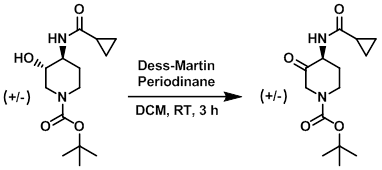
To a solution of the SM (1.30 g, 4.57 mmol) in DCM (200 mL) was added Dess-Martin periodinane (3.88 g, 9.15 mmol). The reaction mixture was stirred at RT for 3 h, after which time it was quenched with 20% aq Na2S2O3 (20 mL) and sat aq NaHCO3 (80 mL). The mixture was stirred until the aq layer was clear. The aq layer was extracted with DCM (3 x 50 mL). The combined organics were dried (Na2SO4) and concentrated to provide the product as a yellow oil. [1.25 g, 97%]
[Patent Reference: WO2014177977, page 75, ![]() (6.0 MB)]
(6.0 MB)]
Example 3
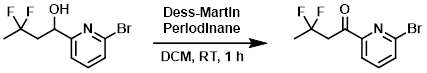
To a solution of the SM (330 mg, 1.2 mmol) in anhydrous DCM (12 mL) was added Dess-Martin periodinane (0.63 g, 1.5 mmol). The reaction was stirred at RT for 1 h. The mixture was diluted with 1:1 10% Na2S2O3/sat aq NaHCO3. The resulting biphasic mixture was stirred for 90 min. To this mixture was added ether (100 mL) and the layers were separated. The org layer was washed with 20 mL of the Na2S2O3/sat aq NaHCO3 solution used above, followed by sat aq NaHCO3, then brine. The org layer was dried (MgSO4) and concentrated to provide the product. [0.33 g, 100%]
[Patent Reference: WO2016011390, page 400, ![]() (20.2 MB)]
(20.2 MB)]
Example 4
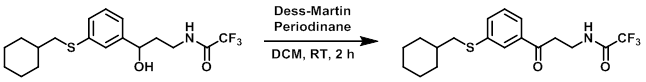
Dess-Martin periodinane (0.861 g, 2.03 mmol) was added under argon to a stirred solution of the SM (0.78 g, 2.08 mmol) in dry DCM. The reaction mixture was stirred at RT for 2 h. The mixture was concentrated in vacuo and the residue purified by flash chromatography (10-40% EtOAc/hexane) to provide to product as a colorless oil. [0.306 g, 39%] [UK Pat App GB2463151A, page 130]
