Grignard
![]()
Common Conditions:
R-MgX + Aldehyde
The reaction of a Grignard reagent (1 equiv) with an aldehyde produces secondary alcohols. The exception being formaldehyde, which gives primary alcohols.[1][3]
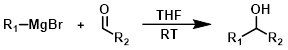
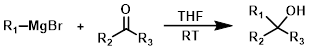
R-MgX + Ketone
The reaction of a Grignard reagent (1 equiv) with a ketone produces tertiary alcohols.[1][3]
R-MgX + Ester
The reaction of a Grignard reagent (2 equiv) with an ester produces tertiary alcohols. Reacting esters with Grignard reagent (1 equiv) to give ketone products is typically difficult because the ketone tends to react further with additional Grignard reagent.[1][2][3]
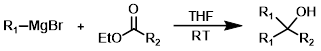
R-MgX + Weinreb Amide
The reaction of a Grignard reagent (1 equiv) with a Weinreb amide produces ketones.[1][2]
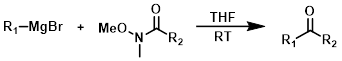
R-MgX + Nitrile (Ketone pdt)
The reaction of a Grignard reagent (1 equiv) with a nitrile, followed by hydrolysis of the reaction mixture produces ketones.[1]
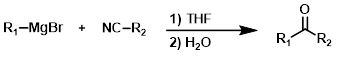
R-MgX + Nitrile (Amine pdt)
The reaction of a Grignard reagent (1 equiv) with a nitrile, followed by reaction with sodium borohydride (NaBH4) provides secondary amines.
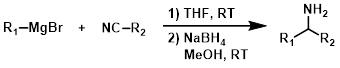
R-MgX + Lactone
The reaction of a Grignard reagent (2 equiv) with a lactone produces diols.[1]
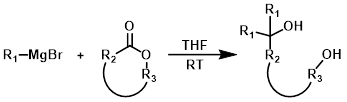
References:
1) Carey, F. A.; Sundberg, R. J.; Advanced Organic Chemistry, Part B: Reactions and Synthesis, 5th Edition
2) Smith, M. B.; March's Advanced Organic Chemistry, 7th Edition
3) Kurti, L.; Czako, B.; Strategic Applications of Named Reactions in Organic Synthesis
