Grignard
(RMgX + Weinreb Amide)
Examples:
Example 1
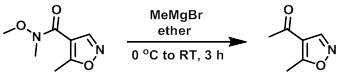
To the SM (480 mg, 2.8 mmol) in dry ether at 0 C was added dropwise a solution of MeMgBr (3M in ether, 2.8 mL, 8.4 mmol). The mixture was slowly allowed to warm to RT and stirred for 3 h. The reaction was quenched with saturated NH4Cl (2 x 20 mL) and extracted with ether. The org layer was dried (Na2SO4) and concentrated to provide the product. [200 mg]
[Patent Reference: WO2010038081, page 135 ![]() (33.8 MB)]
(33.8 MB)]
Example 2
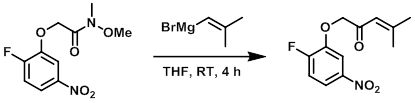
To a solution of the SM (0.32 g, 1.2 mmol) in dry THF (5 mL) at -78 C was added the Grignard Reagent (591 mg, 3.7 mmol). The reaction mixture was stirred at RT for 4 h, after which time it was diluted with H2O (30 mL) and extracted with EtOAc (50 mL). The org layer was dried (Na2SO4) and concentrated in vacuo. The resulting material was purified by column chromatography to provide the product as a yellow colored semi-solid. [140 mg, 43%]
[Patent Reference: WO2014149164, page 337, ![]() (23.7 MB)]
(23.7 MB)]
Example 3
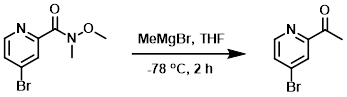
To a solution of the SM (548 mg, 2.2 mmol) in anhydrous THF (25 mL) at -78 C was slowly added MeMgBr (3M in THF, 1.43 mL, 4.4 mmol). The reaction mixture was stirred at -78 C for 2 h, after which time it was quenched with aq NH4Cl and extracted with EtOAc (3 x 30 mL). The combined organics were dried (Na2SO4) and concentrated in vacuo. The resulting material was purified by silica gel chromatography (2:1 PE/EtOAc) to provide the product as a white solid. [330 mg, 75%]
[Patent Reference: WO2016011390, page 149, ![]() (20.2 MB)]
(20.2 MB)]
Example 4
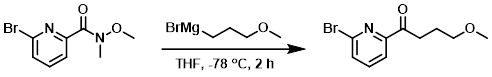
To a solution of the SM (2.5 g, 10.2 mmol) in THF (20 mL) at -78 C was added (3-methoxypropyl)magnesium bromide (2M in THF, 15.3 mL, 30.6 mmol). The reaction mixture was stirred at -78 C for 2 h. Then was added sat aq NH4Cl (10 mL) to quench the reaction. The mixture was extracted with EtOAc (3 x 50 mL). The combined organics were washed with brine, dried (Na2SO4), and concentrated. The residue was purified by silica gel column chromatography (6:1 PE/EtOAc) to provide the product as a light yellow oil. [2.0 g, 76%]
[Patent Reference: WO2016011390, page 308, ![]() (20.2 MB)]
(20.2 MB)]
