Formaldehyde
Other Names:
Formalin
Methanal
Methyl aldehyde
General Information:
Structure:
![]()
CAS Number: 50-00-0
Molecular Weight: 30.03 g/mol
Appearance: Colorless gas (at room temperature)
Formaldehyde is the simplest aldehyde.
Common Uses:
Reagent for reductive methylation (reductive amination) of amines
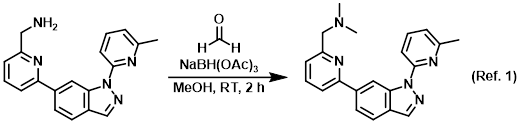
Procedure excerpt:
To a mixture of the SM (115 mg, 0.36 mmol) and formaldehyde (aq, excess) in MeOH (10 mL) was added NaBH(OAc)3 (382 mg, 1.80 mmol) at RT. The reaction mixture . . .
Reagent in Pictet-Spengler reactions
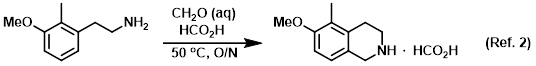
Procedure excerpt:
A solution of the SM (165 g, 1.0 mol) and aq formaldehyde (37 wt%, 30 g, 1.0 mol) in formic acid (1.5 L) was stirred at 50 C overnight. The solvent was removed in vacuo to provide . . .
Reagent in the hydroxymethylation of Grignard reagents to give primary alcohols
Safety:
Formaldehyde is a known carcinogen and is highly toxic to all animals.
References:
1) Patent Reference: WO2016011390, page 65, ![]() (20.2 MB)
(20.2 MB)
2) Patent Reference: WO2016014463, page 91, ![]() (6.7 MB)
(6.7 MB)
3) Coates, R. M.; Denmark, S. E.; Handbook of Reagents for Organic Synthesis, Reagents, Auxiliaries, and Catalysts for C-C Bond Formation
4) Wikipedia: Formaldehyde (link)
5) www.sigmaaldrich.com: Formaldehyde solution - 37 wt. % in H2O (link)
