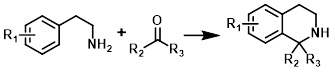
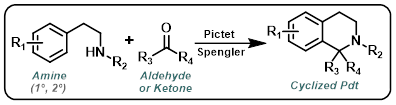
Pictet-Spengler
Common Conditions:
Formaldehyde
Formaldehyde it the simplest aldehyde for Pictet-Spengler reactions. The reactions are typically catalyzed by acid. Aromatic compounds containing EDG's (ex. methoxy) react more readily.[1]
![]()
1,3,5-Trioxane
1,3,5-Trioxane is a stable, solid, easy to handle source of anhydrous formaldehyde. Under acidic conditions 1,3,5-trioxane decomposes to form three molecules of formaldehyde. Aromatic compounds containing EDG's (ex. methoxy) react more readily.[1][2]
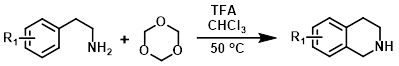
Reaction Map:
The reaction map is intended to provide insight into possible reactions one step before and after the title reaction. It also serves as an alternative way to navigate the website, and as a means of coming up with retrosynthetic ideas. Click on the reaction arrow to visit the page.
| For Beta-arylethylamine: | ||
 |
||
| For Aldehyde: | ||
 |
||
 |
||
 |
||
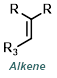 |
||
| For Ketone: | ||
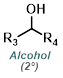 |
||
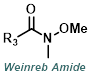 |
||
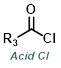 |
||
References:
1) Li, J. J.; Name Reactions in Heterocyclic Chemistry
2) Wikipedia: 1,3,5-Trioxane (link)
