Pictet-Spengler
(1,3,5-Trioxane)
Examples:
Example 1
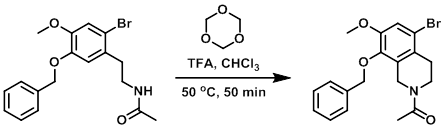
The reaction was carried out 32 times. A mixture of the SM (2.0 g, 5.3 mmol) and 1,3,5-trioxane (2.38 g, 26.4 mmol) in CHCl3 (20 mL) was treated with TFA (10 mL), and the reaction was heated at 50 C for 50 min. The reaction mixture was poured into cooled sat aq NaHCO3 (500 mL) and extracted with DCM (2 x 300 mL). The combined organic layers were washed with sat aq brine (400 mL), dried, and concentrated in vacuo. Purification by silica gel chromatography (20-33% EtOAc/PE) provided the product as a yellow solid. From 1H NMR analysis, the compound was assumed to exist as a mixture of two rotamers. [combined yield: 28 g, 72 mmol, 42%]
[Patent Reference: WO2014177977, page 64, ![]() (6.0 MB)]
(6.0 MB)]
