Reductive Amination
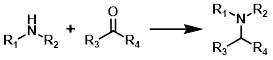
Common Conditions:
NaHB(OAc)3
Sodium triacetoxyborohydride (STAB) is a common reducing agent for reductive aminations. STAB is H2O sensitive and not very compatible with MeOH, therefore reactions are typically done in other solvents (ex. DCE, DCM, THF, or dioxane).
3.png)
NaCNBH3
Sodium cyanoborohydride (NaCNBH3) is not H2O sensitive and is typically used with MeOH as the solvent. Lewis acids [ex. Ti(iPrO)4 or ZnCl2] are sometimes added to improve yields for less reactive substrates.[1][2]
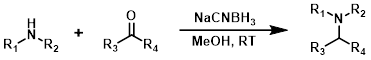
NaBH4
Sodium borohydride (NaBH4) is capable of reducing aldehydes and ketones, therefore NaBH4 is typically only added after sufficient time has been given for complete formation of the imine. Common solvents are MeOH and EtOH.
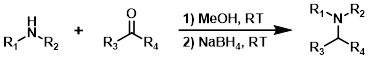
Reaction Map:
The reaction map is intended to provide insight into possible reactions one step before and after the title reaction. It also serves as an alternative way to navigate the website, and as a means of coming up with retrosynthetic ideas. Click on the reaction arrow to visit the page.
| For Amine: | ||
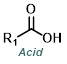 |
||
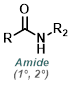 |
||
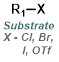 |
||
| For Aldehyde: | ||
 |
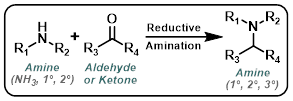 |
|
 |
||
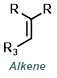 |
||
| For Ketone: | ||
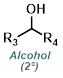 |
||
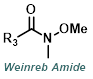 |
||
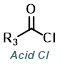 |
References:
1) Burke, S. D.; Danheiser, R. L.; Handbook of Reagents for Organic Synthesis, Oxidizing and Reducing Agents
2) Carey, F. A.; Sundberg, R. J.; Advanced Organic Chemistry, Part B: Reactions and Synthesis, 5th Edition
