Buchwald
Examples:
Example 1
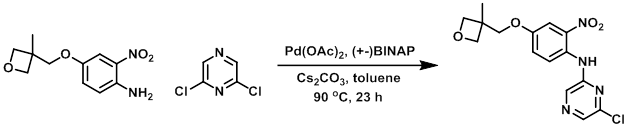
A mixture of the chloride (25 g, 168 mmol), the amine (40 g, 168 mmol), Pd(OAc)2 (1.5 g, 6.72 mmol), (+-) BINAP (4.18 g, 6.72 mmol), and Cs2CO3 (76.7 g, 235.2 mmol) in toluene (800 mL) was stirred at 90 C under N2 for 23 h. The mixture was filtered through a pad of celite and washed with EtOAc. The filtrate was concentrated in vacuo and purified by silica gel flash chromatography (1:5 EtOAc/petroleum ether) to provide the product as a red solid. [22.4 g, 37%]
[Patent Reference: WO2010016005, page 91, ![]() (11.3 MB)]
(11.3 MB)]
Example 2
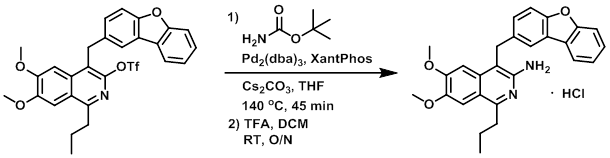
A 2 mL microwave vial under N2 was charged with the SM (13 mg, 0.023 mmol), t-butyl carbamate (5 mg, 0.043 mmol), dry powdered Cs2CO3 (15 mg, 0.046 mmol), Xantphos (4 mg, 0.007 mmol), and Pd2(dba)3 (4 mg, 0.004 mmol). Dry THF (1.5 mL) was then added and the mixture was irradiated in a microwave reactor at 140 C for 45 min, after which time the mixture was diluted with EtOAc (30 mL) and filtered through celite. The solution was washed with brine (10 mL), dried (Na2SO4), and concentrated in vacuo. The residue was then dissolved in DCM (10 mL), treated with TFA (1 mL), and stirred at RT overnight. The resulting mixture was concentrated in vacuo and partitioned between sat aq NaHCO3 (10 mL) and DCM (40 mL). The layers were separated and the org layer was washed with brine (10 mL), dried (Na2SO4), concentrated, and purified by silica gel column chromatography (0-50% EtOAc/cyclohexane) to provide 4 mg of the product as a free-base. The free-base (4 mg) was dissolved in MeOH (2 mL) and treated with a 0.49M HCl solution in MeOH (0.5 mL). The mixture was stirred at RT for 5 min and concentrated in vacuo to provide the HCl salt of the product as a pale brown solid. [4 mg, 38%]
[Patent Reference: WO2012112946, page 187, ![]() (11.2 MB)]
(11.2 MB)]
Example 3

To a solution of the amine (2.81 g, 10 mmol) in dioxane (45 mL) was added the chloride (2.57 g, 9.55 mmol), XantPhos (231 mg, 0.40 mmol), and t-BuONa (1.44 g, 15 mmol). Argon was bubbled through the mixture for 10 min. Pd2(dba)3 (183 mg, 0.20 mmol) was added, and argon was again bubbled through the mixture for 5 min. The reaction mixture was stirred at 100 C for 3 h, after which time it was cooled to 40 C and diluted with DCM (90 mL) and treated with Si-triamine (functionalized silica gel, 2.8 g) overnight at RT. Celite (6 g) was added and the mixture was filtered and the solid was rinsed with DCM (100 mL). The filtrate was concentrated to 25 mL and diluted with 4:1 EtOAc/hexane (20 mL). The resulting slurry was stirred at RT for 5 h, filtered, and the filter cake washed with 1:1 EtOAc/hexane (20 mL). The solids were air dried for several hours to provide the product as an off-white solid. [4.90 g, 100%]
[Patent Reference: WO2012129344, page 126, ![]() (7.3 MB)]
(7.3 MB)]
Example 4
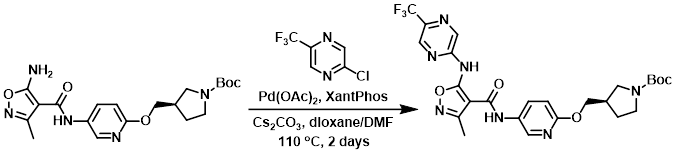
A mixture of the amine (788 mg, 1.9 mmol), the chloride (345 mg, 1.9 mmol), and Cs2CO3 (1.415 g, 4.3 mmol) in 5:1 dioxane/DMF (19 mL) was added to a microwave vial that was flushed with argon. Then, Pd(OAc)2 (42 mg, 0.19 mmol) and XantPhos (109 mg, 0.19 mmol) were added. The vial was sealed and the reaction mixture was stirred at 110 C overnight. Additional chloride (0.33 eq) was added and the reaction mixture was stirred at 110 C for another 24 h. After cooling, the mixture was diluted with 9:1 DCM/EtOH and filtered. The filtrate was concentrated in vacuo and purified by Prep MPLC (Biotage Isolera, 50 g SNAP cartridge, 0-10% EtOH/DCM) to provide the product. [800 mg, 75%]
[Patent Reference: WO2016012477, page 157, ![]() (8.1 MB)]
(8.1 MB)]
