Grignard
(RMgX + Aldehyde)
Examples:
Example 1
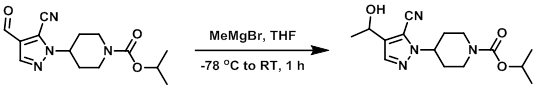
To a solution of the SM (51 mg, 0.18 mmol) in anhydrous THF (2 mL) at -78 C under N2 was added dropwise MeMgBr (3M in ether, 0.070 mL, 0.21 mmol). The cold bath was removed and the reaction was stirred at RT for 1h. The mixture was diluted with 1M aq KHSO4 and extracted with EtOAc (3x). The combined organics were washed with brine, dried (Na2SO4), and concentrated. The resulting material was purified by flash chromatography (eluting with 20-100% EtOAc/heptane) to provide the product as a white solid. [33 mg]
[Patent Reference: WO2012069948, page 64, ![]() (3.9 MB)]
(3.9 MB)]
Example 2
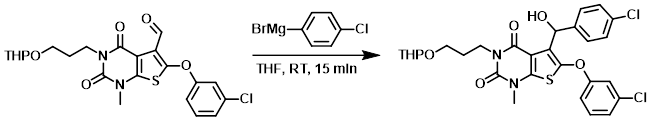
To a solution of the SM (65 mg, 0.136 mmol) in THF (2 mL) was added (3-chlorophenyl)magnesium bromide (35 mg, 0.163 mmol). The reaction mixture was stirred at RT for 15 min, after which time it was diluted with EtOAc (20 mL) and H2O (20 mL). The org layer was dried (Na2SO4) and concentrated. The resulting residue was purified by chromatography (4:1 PE/EtOAc) to provide the product as a yellow oil. [45 mg, 56%]
[Patent Reference: WO2016023832, page 45, ![]() (3.2 MB)]
(3.2 MB)]
