Wittig
[Wittig Reagent (in situ)]
Examples:
Example 1
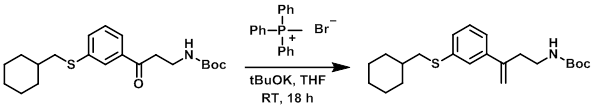
To a suspension of methyl triphenylphosphonium bromide (2.4 g, 6.6 mmol) in THF was added tBuOK (1M in THF, 7.1 mmol) at RT. After stirring for 90 min, a solution of the SM (1.25 g, 3.35 mmol) in dry THF was added. The resulting mixture was stirred at RT for 18 h, after which time it was partitioned between saturated NH4Cl and MTBE. The org layer was dried (Na2SO4), concentrated, and purified by flash chromatography (25-30% EtOAc/hexane) to provide the product as a colorless oil. [0.3 g, 24%] [UK Pat App GB2463151A, page 141]
Example 2
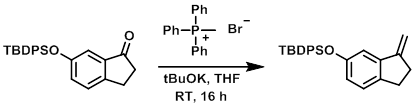
To a solution of the SM (607 g, 1.570 mol) and methyl triphenylphosphonium bromide (673 g, 1.884 mol) in THF (1.000 L) was added tBuOK (1.0M in THF, 1.884 L, 1.884 mol) via addition funnel over 2 h. The reaction mixture was stirred at RT for 16 h, after which time it was concentrated to remove most of the THF. The resulting mixture was suspended in hexane, passed through a pad of silica gel (1 Kg), eluting with hexanes (8 L), then 10% EtOAc/hexane (4 L). The mixture was concentrated to provide the product. [600 g, 99%]
[Patent Reference: WO2010045258, page 80, ![]() (12.0 MB)]
(12.0 MB)]
Example 3
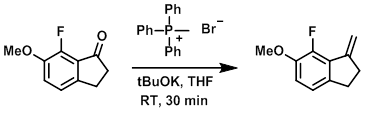
To a solution of the SM (5.22 g, 29.0 mmol) and methyl triphenylphosphonium bromide (12.4 g, 34.8 mmol) in THF (100 mL) was added tBuOK (1.0M in THF, 34.8 mL, 34.8 mmol) via addition funnel over 15 min. The resulting mixture was stirred at RT for 30 min. The mixture was filtered and the filtrate concentrated. The resulting material was purified by chromatography to provide the product as a solid. [3.85 g, 74.6%]
[Patent Reference: WO2010045258, page 82, ![]() (12.0 MB)]
(12.0 MB)]
Example 4
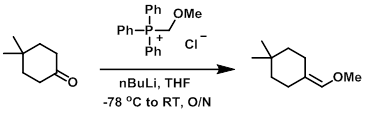
n-BuLi (2.6M in hexane, 2.3 mL, 5.94 mmol) was added dropwise to a stirred solution of (methoxymethyl)triphenylphosphonium chloride (2.04 g, 5.94 mmol) in THF (20 mL) at -78 C. The mixture was stirred at -78 C for 10 min then at RT for 2.5 h. The mixture was cooled again to -78 C and was treated slowly with a solution of the SM (500 mg, 3.96 mmol) in THF (5 mL). The reaction mixture was allowed to warm to RT and stir overnight. The mixture was quenched with sat aq NaHCO3 (20 mL) and extracted with EtOAc. The combined organics were dried (Na2SO4) and concentrated to provide the product as a pale yellow oil which was used for the next step without further purification. [512 mg, crude]
[Patent Reference: WO2015129926, page 71, ![]() (21.5 MB)]
(21.5 MB)]
Example 5
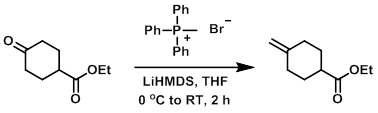
LiHMDS (1.0 M in THF, 15 mL, 15 mmol) was added dropwise to a solution of methyl triphenylphosphonium bromide (5.36 g, 15 mmol) in THF (50 mL) at 0 C. The mixture was stirred at 0 C for 40 min. A solution of the SM (2.04 g, 12 mmol) in THF (20 mL) was then added slowly at 0 C. The reaction mixture was stirred at 0 C to RT for 2 h. The mixture was quenched with sat aq NH4Cl and extracted with hexane. The combined organics were dried (MgSO4) and concentrated in vacuo. The resulting material was diluted with 1:5 ether/hexane (100 mL), stirred for 30 min, and the solids were filtered. The filtrate was concentrated in vacuo and the resulting residue was purified by silica gel chromatography (5% EtOAc/hexane) to provide the product as a colorless oil. [1.478 g, 73%]
[Patent Reference: WO2015129926, page 102, ![]() (21.5 MB)]
(21.5 MB)]
Example 6
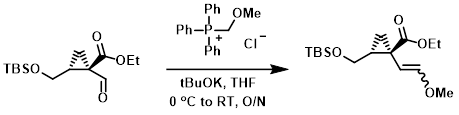
A suspension of (methoxymethyl)triphenylphosphonium chloride (10.4 g, 30.4 mmol) and tBuOK (3.4 g, 30.4 mmol) in THF (120 mL) was stirred at 0 C for 30 min. To this suspension was added dropwise a solution of the SM (10.4 g, 25.3 mmol) in THF (20 mL). The reaction mixture was stirred at 0 C for 1 h, then warmed to RT and stirred overnight. The mixture was diluted with H2O and extracted with EtOAc. The org phase was washed with brine and concentrated. The residue was purified by silica gel flash chromatography to provide the product as a mixture of two isomers. [10.0 g, 90%]
[Patent Reference: WO2016014463, page 63, ![]() (6.7 MB)]
(6.7 MB)]
Example 7
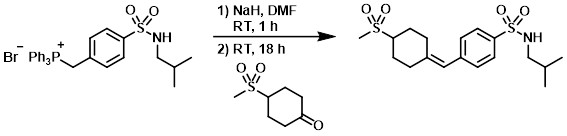
A solution of the SM (1.87 g, 3.29 mmol) in DMF (10 mL) was treated with NaH (60% dispersion in mineral oil, 329 mg, 8.22 mmol) and stirred at RT for 1 h. 1-(Methylsulfonyl)piperidin-4-one (758 mg, 4.28 mmol) was added and the mixture was stirred at RT for 18 h. The mixture was diluted with EtOAc, washed with H2O and brine, dried (Na2SO4), and concentrated in vacuo. The resulting material was purified by silica gel column chromatography (0-100% EtOAc/cyclohexane) to provide the product. [392 mg, 31%]
[Patent Reference: WO2015177325, page 78, ![]() (4.3 MB)]
(4.3 MB)]
Example 8
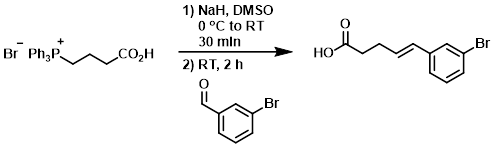
To a solution of the SM (12.87 g, 30 mmol) in dry DMSO (50 mL) at 0 C was added portionwise NaH (3 g, 75 mmol). The reaction mixture was stirred at RT for 30 min, after which time was added dropwise 3-bromobenzaldehyde (5.5 g, 30 mmol). The mixture was stirred at RT for 2 h, then poured into H2O (200 mL) and extracted with EtOAc (100 mL). The aq layer was acidified with conc. HCl and extracted with EtOAc (3 x 200 mL). The combined organics were washed with brine (3 x 100 mL), dried (Na2SO4), and concentrated in vacuo. The residue was purified by silica gel column chromatography (2:1 PE/EtOAc) to provide the product as a yellow oil. [4.4 g, 58%]
[Patent Reference: WO2015089337, page 182, ![]() (17.5 MB)]
(17.5 MB)]
