Ammonium Hydroxide
Other Names:
Aqueous ammonia
Ammonia water
General Information:
Structure:
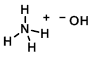
CAS Number: 1336-21-6
Molecular Weight: 35.04 g/mol
Appearance: Colorless liquid
Chemical Formula: NH4OH
Melting Point: -57.5 C (25% w/w)
Boiling Point: 37.3 C (25% w/w)
Density: 0.91 g/mL (25% w/w)
Ammonium hydroxide (NH4OH) solutions are basic, with a 1M NH3 solution having a pH of 11.63. Saturated NH4OH solutions contain 35% ammonia (by mass), however, concentrated solutions that are used in organic chemistry labs are typically 28-30% ammonia (by mass). The concentration of ammonia decreases slightly every time a bottle is opened due to evaporation of ammonia, especially if the bottle was removed from a refrigerator and allowed to warm to room temperature.
Common Uses:
Reagent in amide coupling reactions (serves as a source of NH3)
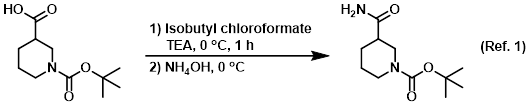
Procedure excerpt:
. . . it was concentrated in vacuo. The resulting material was cooled to 0 C and treated with NH4OH. The resulting solids were filtered, washed with . . .
Nucleophile in SNAr reactions
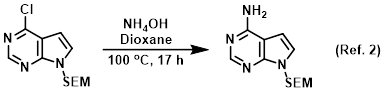
Procedure excerpt:
In a steel pressure vessel was added the SM (3.15 g, 11.1 mmol) and 40% aq NH4OH (21.6 mL, 222 mmol) in dioxane (30 mL) and the mixture was heated . . .
Additive in the catalytic reduction of nitriles to amines
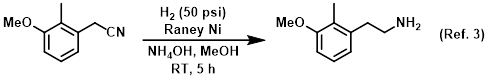
Procedure excerpt:
A mixture of the SM (180 g, 1.10 mol), Raney Ni (40.0 g), and NH4OH (250 mL) in MeOH (1.0 L) was stirred under H2 (50 psi) at RT for 5 h. The mixture was filtered . . .
Additive in silica gel chromatography
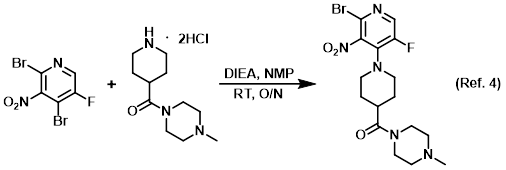
Procedure excerpt:
…concentrated in vacuo. The resulting crude material was purified by chromatography [330 g SiO2, 0-5% (10% NH4OH in MeOH)/(DCM)] to provide . . .
Additive in reverse phase chromatography
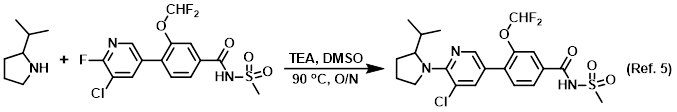
Procedure excerpt:
…heated with shaking at 90 C overnight. The crude solution was purified by reverse phase chromatography (C18, 5-95% ACN/H2O, 0.1% NH4OH) to provide . . .
Safety:
Mixing ammonia containing compounds (ex. NH3, NH4OH, or NH4Cl) and bleach (NaOCl) is very dangerous because it produces toxic by-pdts. People have died from mixing cleaning supplies that contain ammonia and bleach. Household cleaning products that contain ammonia are typically aqueous solutions (NH4OH).
References:
1) Patent Reference: WO2014149164, page 213, ![]() (23.7 MB)
(23.7 MB)
2) Patent Reference: WO2012149280, page 72, ![]() (4.1 MB)
(4.1 MB)
3) Patent Reference: WO2016014463, page 91, ![]() (6.7 MB)
(6.7 MB)
4) Patent Reference: WO2015084384, page 62, ![]() (4.2 MB)
(4.2 MB)
5) Patent Reference: WO2015051043, page 127, ![]() (9.7 MB)
(9.7 MB)
6) Wikipedia: Ammonium hydroxide (link)
7) www.sigmaaldrich.com: Ammonium hydroxide solution (link)
8) The Chlorine Institute, Sodium Hypochlorite Incompatibility Chart (link)
