Sodium Hypochlorite
Other Names:
Bleach
General Information:
Structure:
![]()
CAS Number: 7681-52-9
Molecular Weight: 74.44 g/mol
Appearance: Colorless to yellow/green liquid
Sodium hypochlorite (bleach) is a strong oxidizing agent. Sodium hypochlorite is unstable in solid form and therefore typically exists as a solution. Household bleach is usually about 5% sodium hypochlorite, whereas the reagent grade sodium hypochlorite sold by vendors such as Sigma Aldrich tends to be 10-15%. The pH of commercial bleach is usually 11.0 -12.5.
Common Uses:
Reagent in the oxidation of alcohols to carboxylic acids
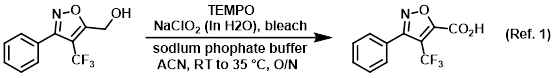
Procedure excerpt:
. . . and sodium phosphate buffer (32.2 mL, 21.59 mmol, 0.67 M) in ACN (30 mL) heated to 35 C. Solutions of NaClO2 (3.91 g, 34.5 mmol) in H2O (4.5 mL) and bleach . . .
Safety:
Mixing sodium hypochlorite (bleach) with ammonia containing compounds (ex. NH3, NH4OH, or NH4Cl) is very dangerous because it produces toxic by-pdts. People have died from mixing cleaning supplies that contain sodium hypochlorite and ammonia. Mixing sodium hypochlorite with acids can be dangerous due to the formation of chlorine gas. Sodium hypochlorite has a strong oxidizing power. Contact with skin and/or eyes can be very harmful.
References:
1) Patent Reference: WO2011017578, page 71, ![]() (8.3 MB)
(8.3 MB)
2) Wikipedia: Sodium hypochlorite (link)
3) www.sigmaaldrich.com: Sodium hypochlorite solution (link)
4) Burke, S. D.; Danheiser, R. L.; Handbook of Reagents for Organic Synthesis, Oxidizing and Reducing Agents
5) The Chlorine Institute, Sodium Hypochlorite Incompatibility Chart (link)
