Alcohol to Acid

Common Conditions:
Jones Reagent
The conditions are quite acidic and may not be compatible with acid-sensitive functionalities. The use of Jones oxidation has declined over time due to the toxic nature of chromium(VI) compounds.[1][2]
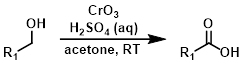
CrO3 (cat.) + H5IO6
A more practical procedure than Jones oxidation is the use of catalytic chromium trioxide (CrO3) with periodic acid (H5IO6). The process requires much smaller amounts of CrO3, which is toxic and environmentally harmful.[1][3]
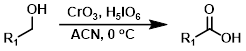
TEMPO (cat.) + NaOCl (cat.) + NaClO2
The procedure uses stoichiometric NaClO2 and only catalytic amounts of TEMPO and NaOCl. The conditions are relatively mild, buffered at pH 6.5 by the sodium phosphate buffer.[4][5]
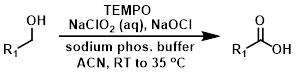
TPAP (cat.) + NMO
TPAP can serve as a mild oxidating agent with high functional group tolerance. The procedure uses a catalytic amount of TPAP in the presence of NMO as a co-oxidant.[1]
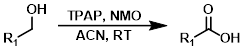
Reaction Map:
The reaction map is intended to provide insight into possible reactions one step before and after the title reaction. It also serves as an alternative way to navigate the website, and as a means of coming up with retrosynthetic ideas. Click on the reaction arrow to visit the page.
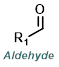 |
 |
|||
 |
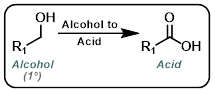 |
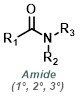 |
||
 |
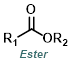 |
|||
 |
 |
References:
1) Tojo, G.; Fernandez, M.; Oxidation of Primary Alcohols to Carboxylic Acids
2) Wikipedia: Jones Oxidation (link)
3) Caron, S.; Practical Synthetic Organic Chemistry
4) Org. Synth. 2005, 81, 195 (link)
5) Wikipedia: Oxoammonium-Catalyzed Oxidation (link)
