Chromium Trioxide
Other Names:
Chromium(VI) oxide
Chromic anhydride
General Information:
Structure:
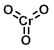
CAS Number: 1333-82-0
Molecular Weight: 99.99 g/mol
Appearance: Red solid
Chemical Formula: CrO3
Melting Point: 196 C
Chromium trioxide (CrO3) is a dark red/orange solid that acts as a strong oxidizing agent. In organic chemistry it is most often associated with Jones oxidation.
Common Uses:
Reagent for the conversion of primary alcohols to carboxylic acids (Jones oxidation)
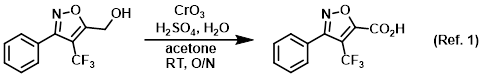
Procedure excerpt:
To an orange, homogeneous solution of CrO3 (12.4 g, 0.123 mol) in H2O (88.4 mL) at 0 C was added H2SO4 (10.8 mL) dropwise via addition funnel over 30 min . . .
Reagent (catalytic) for the conversion of primary alcohols to carboxylic acids
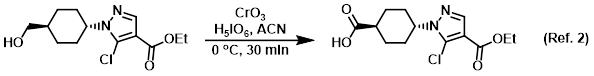
Procedure excerpt:
To a suspension of H5IO6 (159 mg, 0.698 mmol) in ACN was added CrO3 (0.6 mg, 0.0061 mmol), and the mixture was stirred at RT for 30 min. The mixture was cooled . . .
Safety:
Chromium trioxide (CrO3) is highly toxic, corrosive, and carcinogenic. It is a powerful oxidizer which can ignite some materials on contact.
References:
1) Patent Reference: WO2011017578, page 69, ![]() (8.3 MB)
(8.3 MB)
2) Patent Reference: WO2015129926, page 190, ![]() (21.5 MB)
(21.5 MB)
3) Wikipedia: Chromium trioxide (link)
4) www.sigmaaldrich.com: Chromium(VI) oxide (link)
5) Burke, S. D.; Danheiser, R. L.; Handbook of Reagents for Organic Synthesis, Oxidizing and Reducing Agents
