EDC
[1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride]
Other Names:
EDCI
EDAC
General Information:
Structure:
carbodiimide_hydrochloride.png)
CAS Number: 25952-53-8 (HCl salt)
Molecular Weight: 191.70 g/mol (HCl salt)
155.24 g/mol (free-base)
Appearance: White Solid
Melting Point: 110-115 C (HCl salt)
EDC is typically used for coupling reactions in which a carboxylic acid is coupled to an amine. It is usually used as the water soluble HCl salt. EDC is preferred over other carbodiimides because the by-pdt of EDC is water soluble and can be easily removed through an aqueous work-up. EDC is usually used in combination with HOBt.
Common Uses:
Reagent for amide couplings (EDC + HOBt)
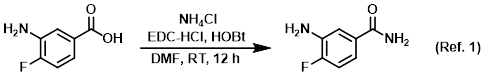
Procedure excerpt:
To a solution of the SM (1.0 g, 6.4 mmol) in DMF (5.0 mL) at 0 C was added EDC-HCl (1.2 g, 7.7 mmol), NH4Cl (1.4 g, 26.9 mmol), and HOBt (1.1 g, 8.3 mmol). The reaction . . .
Reagent for amide couplings (EDC + DMAP)
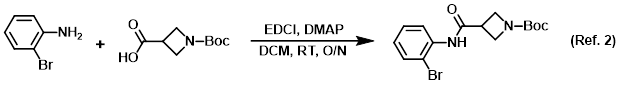
Procedure excerpt:
. . . the amine (150 g, 872 mmol) and DMAP (138.5 g, 1.133 mol) in DCM (2.5 L) was added the acid (872 mmol) in one portion, followed by the addition of EDCI (217 g, 1.133 mol) in . . .
Safety:
EDC is moisture sensitive.
References:
1) Patent Reference: WO2014149164, page 227, ![]() (23.7 MB)
(23.7 MB)
2) Patent Reference: WO2015158653, page 27, ![]() (2.9 MB)
(2.9 MB)
3) Wikipedia: 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (link)
4) www.sigmaaldrich.com: N-(3-Dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (link)
5) Pearson, A. J.; Roush, W. R.; Handbook of Reagents for Organic Synthesis, Activating Agents and Protecting Groups
