Amine to Amide
(EDC + DMAP)
Examples:
Example 1
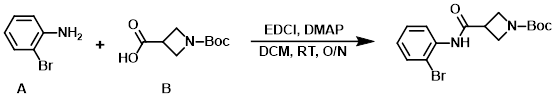
To a stirred solution of the amine (A) (150 g, 872 mmol) and DMAP (138.5 g, 1.133 mol) in DCM (2.5 L) was added the acid (B) (872 mmol) in one portion, followed by the addition of EDCI (217 g, 1.133 mol) in one portion at RT. The resulting mixture was stirred at RT overnight, after which time it was washed successively with 10% aq citric acid, H2O, sat aq Na2CO3, and brine. The org layer was dried (Na2SO4) and concentrated in vacuo to provide the product. [328 g, 85%]
[Patent Reference: WO2015158653, page 27, ![]() (2.9 MB)]
(2.9 MB)]
Example 2
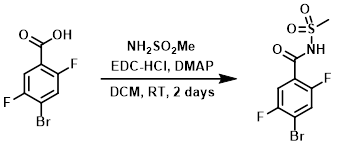
A RBF was charged with the SM (10.0 g, 42.2 mmol), NH2SO2Me (4.82 g, 50.6 mmol), DMAP (11.34 g, 93 mmol), EDC-HCl (16.18 g, 84 mmol), and DCM (150 mL). The reaction mixture was stirred at RT for 2 days. The mixture was concentrated in vacuo to give crude material which was dissolved in DCM (500 mL) and MeOH (10 mL) and filtered through 25 micron filter paper. The crude solution was loaded onto a 1.5 Kg silica gel cartridge (40-63 micron) and was eluted at 300 mL/min over 10 column volumes (2/98/0.1 to 20/80/1 MeOH/DCM/HCO2H) to provide the product. [9.78 g, 73.8%]
[Patent Reference: WO2015051043, page 43, ![]() (9.7 MB)]
(9.7 MB)]
Example 3
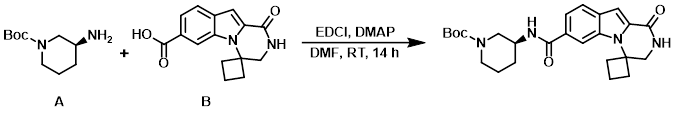
To a mixture of the amine (A) (111 mg, 0.55 mmol) and the acid (B) (150 mg, 0.55 mmol) in dry DMF (5.0 mL) was added DMAP (169 mg, 1.38 mmol) followed by EDCI (213 mg, 1.11 mmol). The reaction mixture was stirred under N2 at RT for 14 h. The mixture was diluted with ice-H2O (50 mL) and extracted with EtOAc (2 x 50 mL). The combined organics were washed with brine (20 mL), dried (Na2SO4), and concentrated. The resulting residue was purified by silica gel column chromatography to provide the product as a white solid. [190 mg, 76%]
[Patent Reference: WO2014149164, page 457, ![]() (23.7 MB)]
(23.7 MB)]
