Substitution (Cl)
(Aromatic Alcohols)
Examples:
Example 1
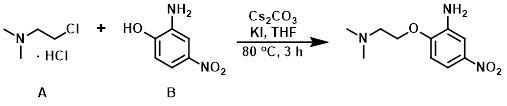
To a solution of the phenol (B) (1.0 g, 6.4 mmol) in THF (30 mL) was added the alkyl chloride (A) (1.1 g, 7.79 mmol), Cs2CO3 (5.2 g, 16.2 mmol), and KI (25 mg, 0.13 mmol). The reaction was stirred at 80 C for 3 h. Upon completion, the reaction mixture was quenched with H2O and extracted with 10% MeOH/DCM. The org layer was dried (Na2SO4), concentrated, and purified by silica gel column chromatography to provide the product as a brown gummy solid. [600 mg, 41%]
[Patent Reference: WO2014149164, page 267, ![]() (23.7 MB)]
(23.7 MB)]
Example 2
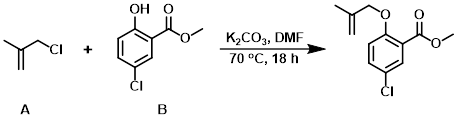
To a solution of the phenol (B) (2.5 g, 13.4 mmol) in DMF (25 mL) was added K2CO3 (2.22 g, 16.1 mmol) and the alkyl chloride (A) (1.46 g, 16.1 mmol). The suspension was stirred to 70 C for 18 h, after which time it was cooled to RT, diluted with H2O (50 mL), and extracted with EtOAc (2 x 25 mL). The combined organics were dried (Na2SO4), concentrated, and purified by chromatography (0-20% EtOAc/hexane) to provide the product.
[Patent Reference: WO2011159297, page 81, ![]() (10.6 MB)]
(10.6 MB)]
